Translate this page into:
Assembling of a cost-effective and adaptable motorised rodent exercise wheel
*Corresponding author: Reshmi R, Department of Physiology, Government Medical College, Thiruvananthapuram, Kerala, India. bineshreshmi@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Reshmi R, Suganthi V, Rajaram S, Malleshappa K. Assembling of a cost-effective and adaptable motorised rodent exercise wheel. Indian J Physiol Pharmacol. 2024;68:64-70. doi: 10.25259/IJPP_558_2023
Abstract
Objectives:
Exercise physiology is one of the leading branches of applied physiology. It is concerned with studies related to the effect of acute and chronic exercise on mental and physical health research in human subjects and animals. There are various methods of physical exercise which have been used in animal studies including rodents. However, the machines available for research purposes are sophisticated and expensive, which also requires an additional annual maintenance cost. The objective is to assemble an efficient, reliable, cost-effective, and humane motorized exercise wheel setup for the study of acute and chronic physical exercise in rodents.
Materials and Methods:
The motorized rodent exercise wheel was assembled using affordable locally available materials.
Results:
A cost-effective, efficient model for rodent exercise was built and the total cost of this setup was 32 USD or 2860 INR only.
Conclusion:
This cost-effective rodent exercise wheel works efficiently for the conduction of exercise-related studies in rodents.
Keywords
Rodents
Aerobic exercise
Motorized rodent exercise wheel
INTRODUCTION
Exercise physiology has been a prominent and evolving leading topic of research for decades. The term ‘exercise’ yields a total number of 576,743 results in PubMed. After using the Boolean operator in PubMed, the search for rodents AND exercise yields 34673 results.
The types of exercises in the rodents include swimming,[1,2] plyometric exercises in jump cages,[3,4] resistance training,[5,6] endurance treadmills training,[7,8] High intensity interval training (HIIT),[9,10] voluntary wheel running and mazes.[11-14] In contrast to conducting experiments on humans, utilizing rodent exercise protocols proves valuable in acquiring tissues for proteomics, gene expression, and receptor expression studies.[15-26] The rodent exercise protocols have also been used for assessing rodent behaviour.[27,28]
Proteomics studies help to understand diseases, cellular processes, and potential drug targets.[15,16] Gene expression studies help researchers to understand how genes are regulated and their various roles in biological processes.[23,24] Receptor expression is important, especially in exercise and cancer research using techniques such as Immunohistochemistry and Western blotting.[29,30]
The various rodent models can incorporate exercise protocols to evaluate rodent behaviour and investigate the impact of exercise across diverse disease conditions. Rodent behaviour studies include studies in social behaviour,[31] cognitive behaviour,[32] emotional behaviour,[33] spatial navigation,[34] sensory perception,[35] drug addiction and reward behaviour,[36] neurological and neurodegenerative diseases,[37] environmental and ecological studies[38] and reproduction and parental behaviour.[39]
The rodents have also been used for understanding the pathophysiology of various diseases such as Crohn’s disease,[40] neurodegenerative diseases,[41] depression,[42] rheumatoid arthritis,[43] diabetes mellitus,[44] Alzheimer’s disease,[45] amyotrophic lateral sclerosis,[46] Parkinson’s disease,[47] inflammatory bowel disease[48] and much more. Various rodent models are available for research, such as immunodeficient mice,[49] oncology mice,[50] humanized mice[51], and transgenic mice.[52]
The main rodent exercising instruments available are treadmills, which are quite expensive. Treadmills are usually preferred as the rodent exercising wheels are not motorized and the exercise will depend on the rodent’s instinct and biological requirement. Besides, it is not able to control the exercise duration, and it requires special housing to install voluntary wheels.
Since the cost of commercially available treadmills may go up to thousands of dollars, we constructed an alternative, a motorized rodent exercising wheel. This instrument helped us to do aerobic exercise in Wistar rats for specified hours in a cost-effective way.
MATERIALS AND METHODS
Figure 1 shows the prototype schematic diagram that was initially designed for the fabrication of the instrument. Figure 2 shows the schematic circuit diagram of motorized rodent exercise wheel. Figures 3-5 show the final product.
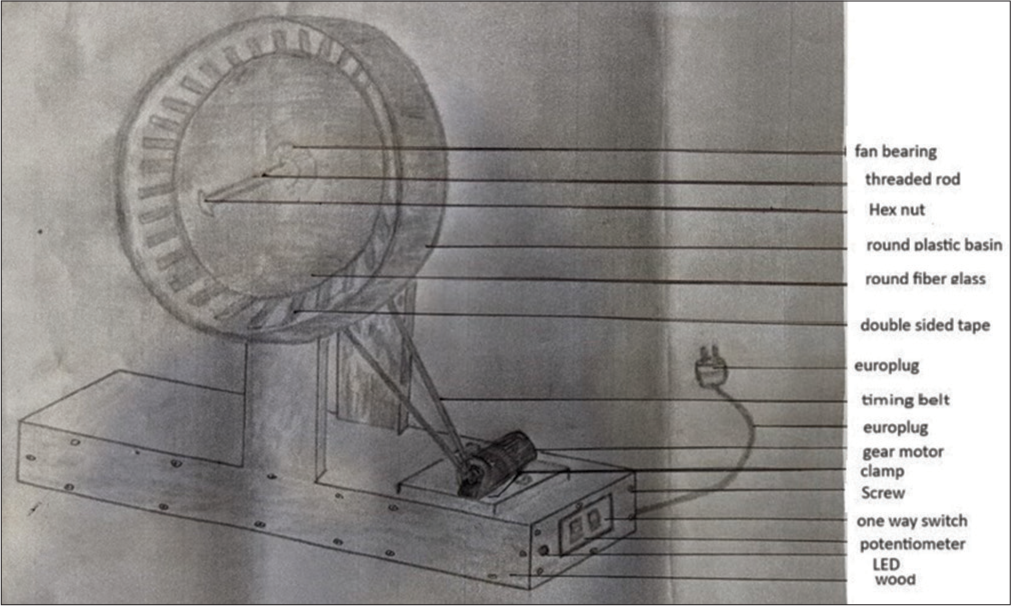
- Prototype schematic diagram of motorized rodent exercising wheel.

- Schematic circuit diagram of motorized rodent exercise wheel.

- This image shows a Wistar rat exercising inside the motorized rodent wheel.

- Full view of the motorized rodent wheel.

- Side view of the motorized rodent wheel.
The materials required for the assembling of the rodent wheel are as follows.
Fan Bearing: Bearings are made up of rolling elements with inner and outer braces for linear or rotating shafts.
Threaded rod and stud bolts: It was used for securing fibre glass.
Hex nuts: Nuts of a hexagonal shape.
Round plastic bin: It was used as the wheel to allow rats to exercise safely.
Round fibre glass: Fibre glass was cut according to the diameter of the plastic bin and attached to the outer diameter of the basin.
Double-sided tape: It was used to form grips on the basin for the rodents to do the exercise. The double-sided tapes were then covered with micropore tapes to prevent damage to the feet of the Wistar rats.
Europlug: It is a flat, two-pole, round-pin domestic AC power plug.
Timing belt: It was used to connect the shaft of the basin to the motor so that the basin would turn according to the speed of the motor.
Gear motor: A gear motor has an electrical motor and a gearbox, which can be used to adjust the speed and increase the torque. It was connected to a potentiometer to adjust the speed.
L clamp: It was used to hold the gearbox onto the wooden platform.
Wooden slabs: The wood used was beech wood. The size of the wooden slabs used was as follows: two pieces of 17 inches by 5 inches, two pieces of 6.5 inches by 5 inches, 12 pieces of 7 inches by 6.5 inches, and two pieces of 15 inches by 4 inches.
The construction of the instrument started with a collection of the above materials. A wooden slab served as a stable platform to support the weight of exercising Wistar rats, preventing any potential tumbling during their workout. It also served as an anchor for the gear motor, which was attached to the wooden slab with the help of clamps. The rod behind the basin was connected to the motor with a timing belt, which was held on a pulley to facilitate the revolving of the basin. The front open end of the basin was secured by a transparent fibre glass connected to the central rod by hex nuts. This ensured the visibility of the exercising rat and also secured the rat inside the wheel. The switch and the regulator for controlling the speed were given on the side of the wooden platform. The inside of the basin was lined with multiple strips of double-sided tape. Each strip was then covered with micropore tape for the animal to get a grip while running.
SCHEMATIC CIRCUIT DIAGRAM OF THE MOTORIZED RODENT EXERCISE WHEEL
Figure 2 shows the schematic circuit diagram of the motorized rodent exercise wheel.
Centre-tapped full wave circuit: A center-tapped full wave rectifier uses a transformer and two diodes to convert the complete AC signal to a DC signal.
Centre-tap transformer: It provides two separate secondary voltages. This type of transformer configuration produces a two-phase, 3-wire supply. The secondary voltages are the same and proportional to the supply voltage so that the power in each winding is the same.
Capacitor: The capacitor is connected parallel to the output of the rectifier in a linear power supply. It decreases the ripple voltage components of the output and increases the DC voltage.
Resistor: The ballast resistor used here is used to limit the current through the LED and to prevent excess current that will damage the LED.
LED: It has been used to identify the on or off position of the switch and to identify the motor speed while adjusting the potentiometer.
Gear motor: A gear motor is designed with an integrated gearbox. Gear motors function as torque multipliers and speed reducers. Hence, it will require less power to move a given load, that is, the weight of the rat.
Potentiometer (regulator): It is connected to the ground (GND) and voltage common collector (VCC). The middle tap provides a voltage between VCC and GND.
One-way switch: This makes or breaks an electric circuit using two terminals.
AC input signal: The AC input voltage is rectified and turned into direct current.
RESULTS
The permission to conduct animal experiments was approved (IAEC/JMMC&RI/06/2019) by the Institutional Animal Ethics Committee (Reg no.1811/PO/Re/S/15/CPCSEA) of the Small Animal Research Facility of Jubilee Mission Medical College, Thrissur, Kerala, India.
Wistar Albino Rats were taken in for doing aerobic exercise using the motorized rodent wheel. The fibre glass on the basin was removed by loosening the hex nuts. One rat was placed inside the basin at a time. Care was taken not to hurt the tails. The fibre glass was then placed over the basin and screwed properly. The motor was then switched on. The rats had to walk as the basin began to turn to retain their horizontal position.
During the initial attempts, we found that the basin was slippery. Hence, it was modified by the addition of double-sided tape covered with micropore on the inner surface of the basin to protect the paws of the exercising animals.
Certain rats attempted to climb the shaft of the basin, prompting us to modify the apparatus by adding a cylindrical cover to the shaft, preventing the rodents from hanging onto the larger cylindrical surface. After the intervention was done, the animals were returned to their housing, having rat feed and RO water.
COST OF THE COMPONENTS FOR MAKING A MOTORIZED RODENT WHEEL
Table 1 shows the cost of the components for making a motorized rodent exercise wheel.
| Components | Cost in Indian rupees |
|---|---|
| Centre tap transformer | 380 |
| Rectifier | 150 |
| Wooden slab | 340 |
| Bearing | 160 |
| Fibre glass | 250 |
| Regulator (potentiometer) | 250 |
| Gear motor | 370 |
| Motor stand | 20 |
| Thread case | 15 |
| Timing belt | 40 |
| Plastic basin | 100 |
| Thread rod | 230 |
| Led | 5 |
| Screws | 30 |
| Europlug | 80 |
| Dimmer | 175 |
| Miscellaneous: Double | 265 |
| stick tape, case, glue, blade, m-seal | |
| Total | 2860 INR/32.24 USD |
IMAGES OF THE INSTRUMENT
Video 1 demonstrates video of Wistar rat exercising in the rodent wheel.
Video 1:
Video 1:Demonstration video of Wistar rat exercising in the rodent wheel.DISCUSSION
This model is very cost-effective and can be made with easily available parts. The rats won’t be injured or exhausted during the experiment, and are more humane. The time required for adaptation to the exercise is very minimal. There is no need to use tail tickling, air puffs or electric shock to instigate the movement as the rats are obliged to move when the wheel starts to turn because it has to keep its horizontal position. The distance covered can be calculated, if required, using the perimeter of the basin and the number of revolutions per minute during each speed.
The limitations of the model are that it can accommodate only one animal at a time. Despite the inclusion of a regulator (potentiometer) in the circuit, its effectiveness was limited by the weight of the animals. The machine overheats after working for about 40 min. Improvisation is necessary to address these issues. Multiple units will have to be constructed for conducting experiments with a larger number of rodents. However, even constructing multiple units will be cost-effective compared to available machines.
CONCLUSION
The rodent exercise studies necessitate the use of highly-priced instruments. However, our endeavor to create a more economical model for rodent exercise has yielded remarkable success. Through innovative approaches and strategic resource allocation, we have managed to develop a cost-effective solution that maintains the integrity and efficacy of the research process while significantly reducing financial barriers. This achievement underscores our commitment to advancing scientific inquiry while promoting accessibility and affordability in experimental methodologies.
Acknowledgment
We express our gratitude to Mr. Arun Das for his assistance in the design of the machine.
Ethical approval
The research/study approved by the Institutional Review Board at Small Animal Research Facility of Jubilee Mission Medical College, Thrissur, Kerala, India., number (IAEC/JMMC&RI/06/2019), dated (IAEC/JMMC&RI/06/2019).
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Videos available on:
Financial support and sponsorship
Nil.
References
- Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848-58.
- [CrossRef] [PubMed] [Google Scholar]
- Swimming exercise and diphenyl diselenide-supplemented diet modulate cerebral cortical and striatal GABA uptake in aged rats. An Acad Bras Cienc. 2022;94:e20200844.
- [CrossRef] [PubMed] [Google Scholar]
- Free-fall landing and interval running have different effects on trabecular bone mass and microarchitecture, serum osteocalcin, biomechanical properties, SOST expression and on osteocyte-related characteristics. Appl Physiol Nutr Metab. 2021;46:1525-34.
- [CrossRef] [PubMed] [Google Scholar]
- Skeletal effects of plyometric exercise and metformin in ovariectomized rats. Bone. 2020;132:115193.
- [CrossRef] [PubMed] [Google Scholar]
- Skeletal muscle metabolomic responses to endurance and resistance training in rats under chronic unpredictable mild stress. Int J Environ Res Public Health. 2021;18:1645.
- [CrossRef] [PubMed] [Google Scholar]
- Resistance-exercise training attenuates LPS-induced astrocyte remodeling and neuroinflammatory cytokine expression in female Wistar rats. J Appl Physiol (1985). 2022;132:317-26.
- [CrossRef] [PubMed] [Google Scholar]
- Idazoxan and efaroxan potentiate the endurance performances and the antioxidant activity of ephedrine in rats. Medicina (Kaunas). 2021;57:194.
- [CrossRef] [PubMed] [Google Scholar]
- Treadmill training reduces cerebral ischemia-reperfusion injury by inhibiting ferroptosis through activation of SLC7A11/GPX4. Oxid Med Cell Longev. 2022;2022:8693664.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of high-intensity interval training (HIIT) on protein expression in Flexor Hallucis Longus (FHL) and soleus (SOL) in rats with type 2 diabetes. J Diabetes Metab Disord. 2022;21:1499-508.
- [CrossRef] [PubMed] [Google Scholar]
- Interactive effects of enalapril administration and novel HIIT wheel-bed training in aged rats. Front Rehabil Sci. 2021;2:764686.
- [CrossRef] [PubMed] [Google Scholar]
- Voluntary wheel running and testosterone replacement increases heart angiogenesis through miR-132 in castrated diabetic rats. Physiol Int. 2019;106:48-58.
- [CrossRef] [PubMed] [Google Scholar]
- Voluntary wheel running during adolescence distinctly alters running output in adulthood in male and female rats. Behav Brain Res. 2020;377:112235.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of ovarian hormones and environment on radial maze and water maze performance of female rats. Physiol Behav. 1999;66:11-20.
- [CrossRef] [PubMed] [Google Scholar]
- Sex differences in the elevated plus-maze test and large open field test in adult Wistar rats. Pharmacol Biochem Behav. 2021;204:173168.
- [CrossRef] [PubMed] [Google Scholar]
- Proteomics and transcriptomics profiling reveals distinct aspects of kidney stone related genes in calculi rats. BMC Genomics. 2023;24:127.
- [CrossRef] [PubMed] [Google Scholar]
- TMT-based proteomics analysis to screen potential biomarkers of acute-phase TBI in rats. Life Sci. 2021;264:118631.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of photobiomodulation and swimming on gene expression in rats with the tibialis anterior muscle injury. Lasers Med Sci. 2021;36:1379-87.
- [CrossRef] [PubMed] [Google Scholar]
- Estrogen deficiency affects tooth formation and gene expression in the odontogenic region of female rats. Ann Anat. 2021;236:151702.
- [CrossRef] [PubMed] [Google Scholar]
- NMDA receptor subunit expression in the supraoptic nucleus of adult rats: Dominance of NR2B and NR2D. Brain Res. 2011;1388:89-99.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of AT1A and AT1B angiotensin II receptor messenger RNA in forebrain of 2-wkold rats. Am J Physiol. 1996;271:E104-12.
- [CrossRef] [PubMed] [Google Scholar]
- Salivary proline-rich proteins: Biochemistry, molecular biology, and regulation of expression. Crit Rev Oral Biol Med. 1993;4:495-502.
- [CrossRef] [PubMed] [Google Scholar]
- Chemical induction of brain tumors in rats by nitrosoureas: Molecular biology and neuropathology. Neurotoxicol Teratol. 1989;11:551-6.
- [CrossRef] [PubMed] [Google Scholar]
- Developmental genetics in emerging rodent models: Case studies and perspectives. Curr Opin Genet Dev. 2016;39:182-6.
- [CrossRef] [PubMed] [Google Scholar]
- Behavioral genetic studies in rats In: Hayman GT, Smith JR, Dwinell MR, Shimoyama M, eds. Rat genomics. Methods in molecular biology. Vol 2018. New York, NY: Springer New York; 2019. p. :319-26. Available from: https://link.springer.com/10.1007/978-1-4939-9581-3_16 [Last accessed on 2023 Oct 16]
- [Google Scholar]
- Biophysical effects, safety and efficacy of raspberry leaf use in pregnancy: A systematic integrative review. BMC Complement Med Ther. 2021;21:56.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of intestinal inflammation by dietary fats. Front Immunol. 2021;11:604989.
- [CrossRef] [PubMed] [Google Scholar]
- Flexibility and rigidity in hunting behaviour in rodents: Is there room for cognition? Anim Cogn. 2022;25:731-43.
- [CrossRef] [PubMed] [Google Scholar]
- The valproic acid-induced rodent model of autism. Exp Neurol. 2018;299:217-27.
- [CrossRef] [PubMed] [Google Scholar]
- Nicotinic alpha 7 receptor expression and modulation of the lung epithelial response to lipopolysaccharide. PLoS One. 2017;12:e0175367.
- [CrossRef] [PubMed] [Google Scholar]
- Expression and localization of the progesterone receptor in mouse and human reproductive organs. J Endocrinol. 2006;191:525-35.
- [CrossRef] [PubMed] [Google Scholar]
- The contagion of social defeat stress: Insights from rodent studies. Neurosci Biobehav Rev. 2020;111:12-8.
- [CrossRef] [PubMed] [Google Scholar]
- Linking mPFC circuit maturation to the developmental regulation of emotional memory and cognitive flexibility. Elife. 2021;10:e64567.
- [CrossRef] [PubMed] [Google Scholar]
- How to study anxiety and depression in rodent models of chronic pain? Eur J Neurosci. 2021;53:236-70.
- [CrossRef] [PubMed] [Google Scholar]
- Interactions between rodent visual and spatial systems during navigation. Nat Rev Neurosci. 2023;24:487-501.
- [CrossRef] [PubMed] [Google Scholar]
- Sensory neuropathy and nociception in rodent models of Parkinson's disease. Dis Model Mech. 2019;12:dmm039396.
- [CrossRef] [PubMed] [Google Scholar]
- Volitional social interaction prevents drug addiction in rat models. Nat Neurosci. 2018;21:1520-9.
- [CrossRef] [PubMed] [Google Scholar]
- Humanized mice for infectious and neurodegenerative disorders. Retrovirology. 2021;18:13.
- [CrossRef] [PubMed] [Google Scholar]
- A review of mammarenaviruses and rodent reservoirs in the Americas. Ecohealth. 2022;19:22-39.
- [CrossRef] [PubMed] [Google Scholar]
- Oxytocin neurons enable social transmission of maternal behaviour. Nature. 2021;596:553-7.
- [CrossRef] [PubMed] [Google Scholar]
- Stem cell therapy for Crohn's disease: Systematic review and meta-analysis of preclinical and clinical studies. Stem Cell Res Ther. 2021;12:463.
- [CrossRef] [PubMed] [Google Scholar]
- Disease-associated oligodendrocyte responses across neurodegenerative diseases. Cell Rep. 2022;40:111189.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic unpredictable mild stress for modeling depression in rodents: Meta-analysis of model reliability. Neurosci Biobehav Rev. 2019;99:101-16.
- [CrossRef] [PubMed] [Google Scholar]
- Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. 2014;506:376-81.
- [CrossRef] [PubMed] [Google Scholar]
- Moringa oleifera Lam in Diabetes mellitus: A systematic review and meta-analysis. Molecules. 2021;26:3513.
- [CrossRef] [PubMed] [Google Scholar]
- Neural stem/progenitor cell therapy for Alzheimer disease in preclinical rodent models: A systematic review and meta-analysis. Stem Cell Res Ther. 2023;14:3.
- [CrossRef] [PubMed] [Google Scholar]
- Meta-analysis of human and mouse ALS astrocytes reveals multi-omic signatures of inflammatory reactive states. Genome Res. 2022;32:71-84.
- [CrossRef] [PubMed] [Google Scholar]
- The DJ1-Nrf2-STING axis mediates the neuroprotective effects of Withaferin A in Parkinson's disease. Cell Death Differ. 2021;28:2517-35.
- [CrossRef] [PubMed] [Google Scholar]
- Targeting uPA-uPAR interaction to improve intestinal epithelial barrier integrity in inflammatory bowel disease. EBioMedicine. 2022;75:103758.
- [CrossRef] [PubMed] [Google Scholar]
- Patient-derived human tumour tissue xenografts in immunodeficient mice: A systematic review. Clin Transl Oncol. 2010;12:473-80.
- [CrossRef] [PubMed] [Google Scholar]
- Mouse models in oncogenesis and cancer therapy. Clin Transl Oncol. 2006;8:318-29.
- [CrossRef] [PubMed] [Google Scholar]
- Humanized mice: A brief overview on their diverse applications in biomedical research. J Cell Physiol. 2018;233:2889-901.
- [CrossRef] [PubMed] [Google Scholar]
- Transgenic mouse models in cancer research. Front Oncol. 2018;8:268.
- [CrossRef] [PubMed] [Google Scholar]







