Translate this page into:
Characterisation of current pharmacotherapeutic COVID-19 clinical trials in India: A registry-based descriptive analysis
*Corresponding author: Arkapal Bandyopadhyay, Department of Pharmacology, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India. drarkapal@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bandyopadhyay A, Choudhary C, Agnihotri A, Handu S. Characterisation of current pharmacotherapeutic COVID-19 clinical trials in India: A registry-based descriptive analysis. Indian J Physiol Pharmacol 2021;65:141-5.
Abstract
COVID-19 has emerged as a global pandemic. There is currently a spurt of clinical research to generate evidence to treat this novel disease. The aim of the current study is to characterise the pharmacotherapeutic intervention-related clinical trials registered in India. COVID-19 clinical trials registered in India were analysed from data retrieved from Clinical Trial Registry-India, ClinicalTrials.gov and International Clinical Trial Registry Platform. Parameters such as study design, sample size, pharmacotherapeutic interventions and primary outcomes were evaluated. A total of 267 studies were screened among which 103 clinical trials were assessed for descriptive analysis. Majority of registered trials (55.3%) were in Indian System of Medicine followed by antimalarials such as hydroxychloroquine/chloroquine (10.5%) and plasma therapy (7.1%). Most commonly used study design was randomised parallel group in 63%. Open-label study was seen in 47.5%. Primary outcome was improvement of clinical symptoms in 46.6% followed by rate and time of viral load reduction in 18.2%. Maharashtra (n = 35) followed by Delhi (n = 29) had the maximum number of registered trial sites. The study gives broad perspective on the current trend of clinical trials on COVID-19 being conducted in India. The outcomes of these trials will lead to generation of valuable data for evidence-based treatment of COVID-19.
Keywords
COVID-19
Clinical trials
Pharmacotherapy
Registry
INTRODUCTION
COVID-19 pandemic which emerged from Wuhan (China) in December 2019 had spread worldwide impacting the health of humans globally.[1] Clinical manifestations range from asymptomatic to mild fever, cough, running nose and dyspnoea.[2] Patients occasionally progress to pneumonia with acute respiratory distress syndrome and death. Older age and associated comorbidities usually lead to a progressive disease and mortality.[3] The World Health Organisation (WHO) had declared it as a global pandemic on 11 March 2020.[4]
On 30 January 2020, India reported the first confirmed case of COVID in Kerala. The disease gradually spread throughout the entire nation. States like Maharashtra followed by Tamil Nadu, Delhi and Gujarat reported maximum number of cases till date.[5,6]
Research is being conducted to develop effective therapeutic options for COVID-19. Clinical trials benefit patients, improve therapeutic regimens and ensure advancement in medical practice.[7] Registration of the clinical trials is the first step toward potential transparency and proper dissemination of clinical research outcomes to generate evidence for further research and therapeutic decisions. Registry ensures the ethical functioning of trial so that information about ongoing and previously conducted trials are easily accessible.[8]
Launched in September 2008, ClinicalTrial.gov is the world’s largest registry of clinical trials. It requires the submission of basic details and results of the clinical trials. Results of the study include participant flow, baseline characteristics, outcomes measures and statistical analysis.[9] Clinical Trails Registry-India (CTRI) was launched in 2007.[10] The site is hosted by National Institute of Medical Statistics a branch of Indian Council of Medical Research (ICMR). It is a free and online public record system for registration of clinical trials that are being conducted in India. The WHO’s International Clinical Trials Registry Platform (ICTRP) is open database containing the basic set of information deemed essential to describe a clinical study. The study records in the database are openly accessible with a unique ISRCTN identification number.[11]
Conducting a well-designed clinical trial during a pandemic is a challenging task. Rigorous methodology analysis, best ethical practices and timelines need to be considered. To date, information regarding basic characteristics and design methodologies of registered trials on COVID-19 is scarce. It was seen that limited relevant literature of clinical trial methodology on COVID-19 has been published. To review current practices and identify knowledge gaps in designing pharmacotherapeutic clinical trials on COVID-19, we planned to extract relevant set of registered trials and summarise their key characteristics and design issues. We further analysed the test drugs, outcomes and disease severity among other parameters.
MATERIALS AND METHODS
Search strategy
The key words used for search strategy in the present study were ‘COVID-19,’ ‘SARS-COV-2’ and ‘India.’ Efforts were made to search for all COVID-19 clinical trials registered from India on CTRI, ClinicalTrials.gov and ICTRP till 24 July 2020. All retrieved records were downloaded, and the data regarding study ID, study title, sample size, drug categories, trial design, trial groups and type of participant enrolled were collected. Studies were analysed and duplicates were excluded in final analysis. A standard Microsoft Excel database was created for study analysis.
Selection criteria
The registered trial was considered for selection if the trial met the following inclusion criteria: (1) Mention of pharmacologic intervention and (2) trial is conducted in India. Non-pharmacological interventions and observational studies were not included in the final analysis.
Statistical analyses
Categorical variables are expressed as percentages and continuous values were expressed as median and interquartile range. All statistical procedures were performed using the STATA software (Version-15).
RESULTS
COVID-19 trials registered in India
A total of 267 studies (164 in CTRI, 89 in ICTRP and 14 in ClinicalTrial.gov) were retrieved from the database on 24 July 2020. Observational studies (n = 95) and duplicates (n = 61) were excluded from the analysis. Among the intervention-related trials, there were eight non-pharmacological intervention trials and hence were not included in the final analysis [Figure 1].
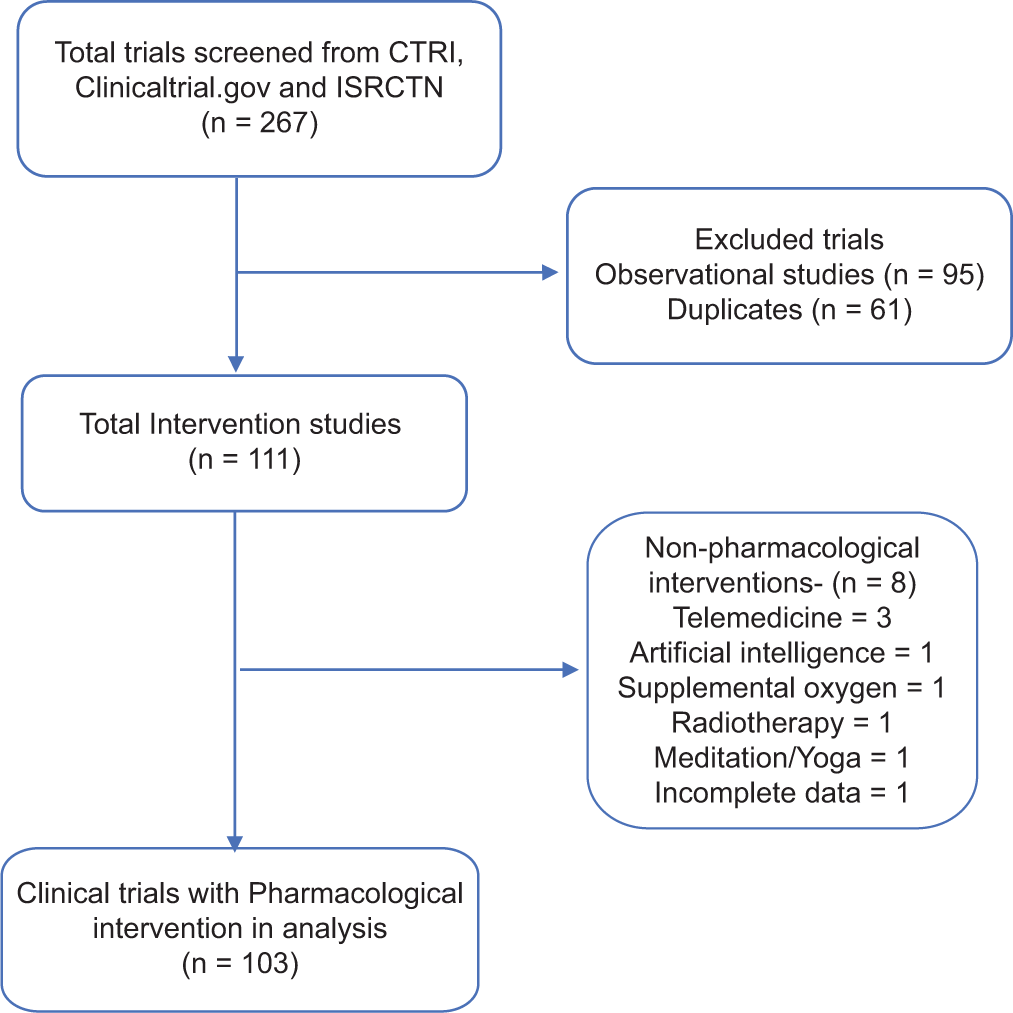
- Flowchart of search strategy and screening COVID-19-related pharmacological intervention trials.
Characteristics of COVID-19 trials in India
Pharmacological intervention clinical trials were stratified and categorised according to drug category. Indian System of Medicine (Ayurveda, Yoga and Naturopathy, Unani, Siddha and Homoeopathy (AYUSH) constituted majority (55.3%) of registered trials followed by antimalarial drugs (10.5%), convalescent plasma therapy (7.1%) and vaccines and biologicals (6.2%) trials [Table 1].
| Drug category | n(%) |
|---|---|
| Ayurveda, Yoga and Naturopathy, Unani, Siddha and Homoeopathy | 62 (55.3) |
| Antimalarial | 12 (10.5) |
| Plasma | 8 (7.1) |
| Vaccine/biological | 7 (6.2) |
| Antiviral | 6 (5.3) |
| Antineoplastic/immunomodulators | 6 (5.3) |
| Antiparasitic | 5 (4.4) |
| Supplements (Vitamin C, zinc, thioxanthine, theaflavin and polyphenols) | 3 (3.65) |
| Antihypertensive | 1 (0.8) |
| Glucocorticoids | 1 (0.8) |
| Local anaesthetic | 1 (0.8) |
| Antiseptics | 1 (0.8) |
AYUSH: Ayurveda, Yoga and Naturopathy, Unani, Siddha and Homoeopathy
Sample size of the analysed studies was highly variable. A total of 287,831 participants were planned to be recruited in clinical trials. Thirty-seven trials had planned to recruit less than 100 participants, 38 trials planned to recruit 100– 500 participants whereas 28 trials planned to recruit more than 500 participants. The range of participants varied from 6 (CTRI/2020/05/025432 – Phase 1 trial on Cytokine cocktail Therapy) to 50,000 (CTRI/2020/05/025171 – trial on Ayurvedic preparation – Samshamani Vati, anu taila and turmeric).
Among the AYUSH trials registered sample size ranged from 30 to 50,000 participants. Median sample size was 120 (IQR: 60–1200). Median sample size in trial of hydroxychloroquine-based regimen and antiviral drugs therapies was 127.5 and 100, respectively.
Adaptive design was used in three studies where interventions were open-label Ayurvedic therapy with Bryonia alba (CTRI/2020/06/025558), Guduchi Ghan Vati (CTRI/2020/05/025370) and recombinant BCG vaccine in reducing infections among high-risk patients (CTRI/2020/04/024749).
Trials designs are depicted in [Figure 2]. The most common design used in the study was randomised parallel group (63%). While assessing blinding, observed data showed open label in 49 (47.5%), double blind in 13 (12.6%) and single blind in 6 (5.8%) trials. Blinding details were not provided in 33.9% of registered trials.
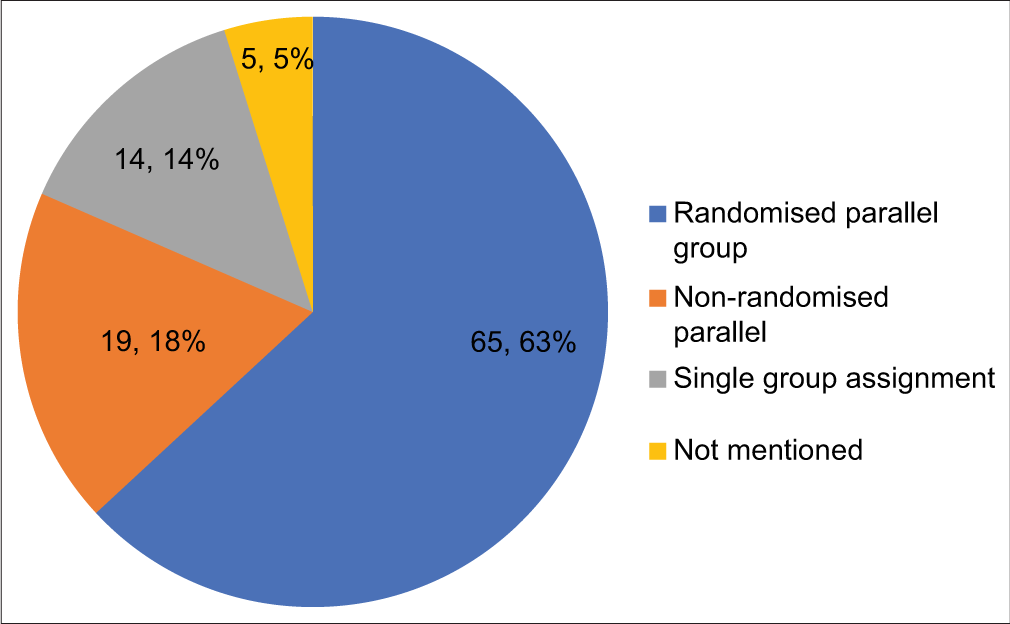
- Distribution of the registered trials by types of trial designs.
Overall, 89 (86.4%) had two groups, 5 (4.8%) each had single group and triple group trials. Only 3 (2.9%) studies had four arms and 1 (0.9%) had five arms. Thirty-one trials (30.1%) had planned to recruit healthy volunteers for their research. Among the trials who included COVID-19-positive patients’, seriousness of disease was not mentioned in 69 (66.9%) trials whereas 2 (1.94%) trials included mild-to-moderate patients and 1 (0.9%) trial planned to recruit only severe cases. Funding agencies commonly involved were medical college/ university (government – 32.3% and private – 16.1%), government bodies like ICMR (19.1%) and industrial sponsored studies (25.2%). Private hospitals were funding sources in 4%. Other sources of funding were mentioned in 3.03%.
Most frequently used study duration was 6 months in 30 (29.1%) trials followed by 3 months in 29 (28.1%) and 12 months in 16 (15.5%) trials. Improvement in clinical symptoms was the primary outcome in majority of registered clinical trials (46.6%) followed by rate and time of viral load reduction in 18.2% [Table 2]. Maharashtra (n = 35), followed by Delhi (n = 29), Uttar Pradesh (n = 17), Madhya Pradesh (n = 13), Tamil Nadu (n = 13), Karnataka (n = 12), Gujarat (n = 10) and Telangana (n = 10), had majority of registered trial sites across India [Figure 3].

- Distribution of registered trials by state.
| Primary outcomes | n(%) |
|---|---|
| Improvement of clinical symptoms | 110 (46.6) |
| Rate and time of viral load reduction | 43 (18.2) |
| Incidence of COVID-19 infection | 23 (9.75) |
| Reduction in mortality rate | 21 (8.9) |
| Progression/severity of disease | 15 (6.3) |
| Reduced hospital stays | 8 (3.3) |
| Potential effect of drug use | 7 (2.97) |
| Improvement in oxygen saturation | 5 (2.1) |
| Questionnaire based | 2 (0.8) |
| Side effects | 1 (0.4) |
| Pharmacokinetic study | 1 (0.4) |
DISCUSSION
India has faced various outbreaks/epidemics (such as H1N1, H5N1, avian influenza, Zika, Ebola, Nipah and SARS) in the past decade. They were successfully tackled with appropriate clinical research and effective management.[12] COVID-19 pandemic has led to the spurt of clinical trials globally. Since advent of disease, the research opportunities have expanded in an attempt to develop a potential therapeutic target to treat this virus. Research on treatment, prevention of disease and vaccine candidates’ molecules are of utmost importance in this crisis situation. ICMR had propagated patient safety to be of utmost importance in COVID-19 trials.[13]
Antimalarial drugs such as hydroxychloroquine and chloroquine are thought to be effective in the prophylaxis and treatment of COVID-19 infection.[14] Low cost and widespread availability of these drugs in the developing countries like India are a major advantage for its usage. ICMR has recommended its use in prophylaxis and treatment of COVID-19 infection. A study by Chatterjee et al. reported that healthcare workers who had taken more than 4 doses of hydroxychloroquine had reduced chances of SARS-CoV-2 infection.[15] Increased mortality and cardiotoxic side effects reported in some studies although most have methodological limitations.[16,17]
Antiviral drugs such as lopinavir-ritonavir, ribavirin, remdesivir (CTRI/2020/04/024773) and favipiravir (CTRI/2020/05/025114) have been proposed to be beneficial in COVID-19 infection.[18,19] Remdesivir, a product of Gilead sciences, has shown a lot of promise in treatment of severe stage of this infection.[20] The USA and India government has given emergency approval to remdesivir for the treatment of severe COVID-19 infection. Trials conducted have shown to decrease mortality in patients with severe disease.[21,22] A randomised control trial showed faster time to clinical improvement but 12% of patients had to stop the drug due to adverse reactions.[23]
Clinical trials hold the key to generating evidence in crisis time of pandemic. Conducting randomised clinical trials in times of pandemic is particularly difficult and challenging although it has minimal bias and provides highest level of evidence among clinical studies. Our studies showed that 63% of all interventions were randomised control trials although blinding details were not given in 33.9%. A similar registry analysis by Huang et al. in China analysed 262 intervention trials with 76% randomised parallel group design.[10]
Our study showed a focus on exploring the Indian system of medicine as a therapeutic option for COVID-19. Nearly 55.3% of the registered trials are related to AYUSH derivatives. Homoeopathic medicine preparations were most commonly studied. The trend was similar to a study by Huang et al. where traditional Chinese medicines trials constituted 27.9% and another study by Lu et al. during early days of COVID infection constituted 35.5%.[23]
India currently has multiple ongoing clinical trials of varying sample sizes. A proper conduct and evaluation of such trials will help in identifying suitable drugs to treat COVID-19. Considering trial designs, majority of the trials were open-label randomised controlled trials. Almost 30% of studies were on healthy volunteers. Hence, diligent conduct of such studies will lead to fruitful results with minimal bias.
As the current pandemic is on the rise in India, it may be difficult for researchers to properly adhere to recommended guidelines. Scientific conduct of the study, unbiased evaluation and proper interpretation of results will lead to the development of effective treatment guidelines for COVID-19 infection. The efficacy and safety of this trial can provide crucial evidence in the fight against COVID-19.
CONCLUSION
This study provides an outlook into current scenario of trials conducted in India. A significant focus has been given to the Indian system of medicine in the symptomatic as well as definitive management of COVID-19. The proper conduct of trials will lead to opening up of avenues for successful treatment of COVID-19 infection. These studies once conducted, might be helpful in forming a backbone for the development of future research hypothesis in combating the infection. It is imperative to form collaborations between research institutes and various public health agencies for successful dissemination of study findings to overcome the pandemic.
Contributions
AB proposed the idea. CC, AA and AB did extensive literature search on the topic. AB, CC, AA and SH contribute to development of the manuscript. AB and SH did final editing and moderation. All authors have seen the manuscript and agree to the content and data. All the authors played a significant role in the paper.
Ethics statement
The article does not have participation of any human being and animal.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- WHO Coronavirus Disease (COVID-19) Dashboard. 2020. Available from: https://www.covid19.who.int [Last accessed on 2020 Jul 17]
- [Google Scholar]
- Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 and tuberculosis co-infection: A neglected paradigm. Monaldi Arch Chest Dis. 2020;90:518-22.
- [CrossRef] [PubMed] [Google Scholar]
- COVID19 India. 2020. Available from: https://www.covid19india.org [Last accessed on 2020 Jul 18]
- [Google Scholar]
- COVID-19 State Data. 2020. Available from: https://www.mohfw.gov.in [Last accessed on 2020 Jul 18]
- [Google Scholar]
- Clinical Trails Registry-India. 2020. Available from: http://www.ctri.nic.in/clinicaltrials/login.php [Last accessed on 2020 Jul 17]
- [Google Scholar]
- Registering a clinical trial in ClinicalTrials.gov. Chest. 2007;131:909-12.
- [CrossRef] [PubMed] [Google Scholar]
- About the Result Database. 2020. Available from: https://www.clinicaltrials.gov/ct2/about-site/results [Last accessed on 2020 Jul 18]
- [Google Scholar]
- Characteristics of COVID-19 clinical trials in China based on the registration data on ChiCTR and ClinicalTrials.gov. Drug Des Devel Ther. 2020;14:2159-64.
- [CrossRef] [PubMed] [Google Scholar]
- ISRCTN Registry. 2020. Available from: https://www.isrctn.com [Last accessed on 2020 Jul 17]
- [Google Scholar]
- Ethics preparedness for infectious disease outbreaks research in India: A case for novel Coronavirus disease 2019. Indian J Med Res. 2020;151:124-3.
- [CrossRef] [PubMed] [Google Scholar]
- National Guidelines for Ethics Committee Reviewing Biomedical and Health Research during COVID-19 Pandemic. 2020. Available from: https://www.icmr.gov.in/pdf/covid/techdoc/ec_guidance_covid19_06052020.pdf [Last accessed on 2020 Jul 17]
- [Google Scholar]
- Therapeutic opportunities to manage COVID-19/SARSCoV-2 infection: Present and future. Indian J Ophthalmol. 2020;68:693-702.
- [CrossRef] [PubMed] [Google Scholar]
- Healthcare workers and SARS-CoV-2 infection in India: A case-control investigation in the time of COVID-19. Indian J Med Res. 2020;151:459-67.
- [CrossRef] [PubMed] [Google Scholar]
- Hydroxychloroquine in patients with COVID-19: A systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14:589-96.
- [CrossRef] [PubMed] [Google Scholar]
- An updated systematic review of the therapeutic role of hydroxychloroquine in Coronavirus disease-19 (COVID-19) Clin Drug Investig. 2020;40:591-601.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacologic treatments for Coronavirus disease 2019 (COVID-19): A review. JAMA. 2020;323:1824-36.
- [CrossRef] [PubMed] [Google Scholar]
- Antiviral treatment of COVID-19. Turk J Med Sci. 2020;50:611-9.
- [CrossRef] [PubMed] [Google Scholar]
- Compassionate use of remdesivir for patients with severe COVID-19. N Engl J Med. 2020;382:2327-36.
- [CrossRef] [PubMed] [Google Scholar]
- Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569-78.
- [CrossRef] [Google Scholar]
- An evidence mapping and analysis of registered COVID-19 clinical trials in China. BMC Med. 2020;18:167.
- [CrossRef] [PubMed] [Google Scholar]






