Translate this page into:
EEG cortical sources of face and word as distractors during emotional interference
*Corresponding author: Simran Kaur, Stress and Cognitive Electroimaging Lab, Department of Physiology, All India Institute of Medical Sciences, Delhi, India. drsimrankaur@aiims.edu
-
Received: ,
Accepted: ,
How to cite this article: Batabyal T, Muthukrishnan SP, Tayade P, Sharma R, Kaur S. EEG cortical sources of face and word as distractors during emotional interference. Indian J Physiol Pharmacol 2023;67:172-80.
Abstract
Objectives:
Faces and words bearing emotional valence are commonly encountered affective stimuli by humans. It is known that emotional distractors interfere with the task goal and disrupt the cognitive performance. However, the differential cortical sources involved as neural substrates, to process emotional distractors, while affecting performance during the presence of another emotional stimuli, (emotional interference) is still unexplored.
Materials and Methods:
Emotional interference was studied in 20 healthy adults (25.15 ± 2.978) while performing face-word interference (FwI) and Word-face interference (WfI) tasks, wherein words with emotions and faces were distractors and 128 channel electroencephalogram was acquired simultaneously. Further, standardised low-resolution electromagnetic tomography-based source analysis was done to study the differences in the cortical activity between the tasks across 66 gyri.
Results:
Higher reaction time (RT) and lower accuracy percentage were seen for categorising face in presence of word (FwI) versus word in presence of face (WfI) (P < 0.001). We observed no difference in RT and lower accuracy percentage between incongruent FWI trials as compared to congruent FWI trials. In addition, we observed higher RT and lower accuracy percentage between incongruent WFI trials as compared to congruent WFI trials. Source analysis revealed higher cortical activity across 60 gyri and significantly lower cortical activity across three gyri during performance of FwI compared to WfI task (P < 0.05/66). Activation of areas associated with saliency, conflict and goal directed behaviour exhibited consistently higher activity in FwI trials compared to WfI trials indicating the robustness of face processing and the hierarchical interplay of neural substrates in conditions of cognitive control.
Conclusion:
Cortical processing of face emerged as more potent distractor compared to an emotional word during emotional interference task.
Keywords
Emotional interference
Faces
Words
EEG
Cortical sources
INTRODUCTION
Over the decades, human brain has evolved with dedicated neural architecture and adopted efficient communication strategies to rapidly process emotional stimuli. Higher centres influence the attentional resources as an adapted strategy to approach or avoid an appetitive or aversive emotional stimulus.[1,2] Unconscious detection of a cue which requires minimal attention is a cornerstone of automatic processing, especially during processing of an emotional stimuli. Furthermore, when the emotional stimuli are repeated or with experience, there occurs faster processing.[3] In addition, survival-related emotional stimuli are efficiently processed when compared to the other emotional stimuli.[4]
Previous functional magnetic resonance imaging (fMRI) studies have proposed that neural projections from amygdala contribute to rapid evaluation and automatic processing of affective stimuli.[5] However, the role of cognitive control emerges especially in a choice-based decision-making while processing two different emotional cues.
Recently, we proposed that emotional interference condition does disengage the areas associated with frontoparietal attentional network, default mode network and engages premotor cortical areas as compared to baseline.[6] However, it intrigued us further to understand how this phenomenon takes place when two different emotional stimuli compete for limited attentional resources at the backdrop of a cognitive task at hand. Faces and words bearing emotional valence are two commonly encountered affective stimuli by humans on day-to-day basis. The skill required for the recognition of the facial emotion is acquired as early as during infancy and tend to become an overlearned skill through repeated exposure. Due to experience, a literate adult can read effortlessly and in fact it takes an effort to curb reading than to read.[7] Although unconditioned evaluation of affective face and word is well documented, yet the underlying neural substrates associated with processing are yet to be explored.
Emotional interference has been explored using fMRI in depression, wherein higher activity in the left rostral anterior cingulate cortex (ACC) and right precuneus was reported during presentation of sad words.[8] In addition, Dresler et al. reported higher left frontal gyrus activity in patients with panic disorder patients during exposure to panic words compared to neutral words during Stroop task.[9] Recently, Khanna et al. explored cortical sources of emotional interference using magnetoencephalography and reported higher theta activity in prefrontal and superior temporal cortices in veterans without post-traumatic stress disorder (PTSD) compared to the ones with PTSD.[10] The phenomenon of emotional interference has been explored using emotional words and faces by a study by Ovaysikia et al. in 2011. They explored the neural substrates for cognitive control during emotional interference using fMRI in healthy subjects and reported activation of medial prefrontal gyrus during incongruent trials compared to congruent trials.[11]
Electroencephalogram (EEG) is a non-invasive tool which can be used to envisage neural processing, at milliseconds temporal resolution. Thus, the present study attempts to unravel hierarchical processing and neural substrates during Face-word and Word-face emotional interference task using high density single trial quantitative EEG. Further, the cortical sources during performance of FwI versus WfI were elucidated across 66 gyri using source analysis.
MATERIALS AND METHODS
Study design
This was a cross-sectional study, conducted at stress and cognitive electroimaging laboratory, Department of Physiology, All India Institute of Medical Sciences, New Delhi. The protocol was approved by the institutional ethics committee (IECPG-214/24.02.2016) and was performed with the ethical standards laid down in Declaration of Helsinki.
Participants
Twenty right-handed healthy volunteers (ten males and ten females) aged 25.15 ± 2.978 years were recruited for the present study based on convenient sampling. The young adults (18–40 years) of either gender, with normal vision or corrected visual acuity of 6/6, who could read English language were included for the study, while the subjects with past or present history of neuropsychiatric/neurodegenerative disorder or colour blindness were excluded from the study.
Study protocol
Once the participants came to the laboratory, they were acquainted with the experiment and written informed consent was obtained. The subjects were tested for handedness using Edinburgh’s Handedness Inventory.[12] After, familiarising the subjects with the task with practice trials, emotional interference task was administered (as explained below) and simultaneously EEG was acquired.
Emotional interference task
The phenomenon of emotional interference was studied experimentally using ‘Emotional Interference task.’ The task was presented using 17-inch LCD display, placed 70 cm across the subject. The task was designed using E Prime version, 2.0., wherein stimuli comprised of thirty international affective picture system pictures (IAPS) of human depicting facial emotions (ten IAPS pictures each of negative, positive and neutral (negative < 4, neutral 4.5 < 6 and positive 6.5 >) valence and high arousal (rating > 6) and three emotional words from Affective Normative English Words that is, HAPPY (positive valence), SAD (negative valence) and BLAND (neutral valence).[13] The words were placed horizontally over the IAPS picture of the face such that emotion of the face can be recognised well by the subjects using Adobe Photoshop. The text was written using red colour, since it is documented to be less arousing and more pleasing.[14]
Two types of variants of the emotional interference task were designed (Face-word interference [FwI] and Word-face interference [WfI]) which differed in terms of the instruction given.
FwI
A FwI task (also called Face word emotional interference task) has been designed using Eprime V2. The instruction for the participant was to categorise the facial emotion of the image (target) using key press and ignore the emotional category of the word overlaid on it (distractor). [Figures 1 and 2] serve as examples of the instructions and associated stimuli used during FwI task trials. As shown, first trial of the FwI task required the subject to ignore the emotional category of the word ‘HAPPY’ serving as a distractor and press key 1 for the positive facial emotion. As a distraction in the second trial, the emotional category of the word ‘BLAND’ should not be focused on; instead, key 2 should be pressed for the negative facial expression. Similarly, during the third trial, key 3 should be pressed to focus on the neutral facial expression while ignoring the emotion of the word ‘SAD,’ which serves as a distractor.
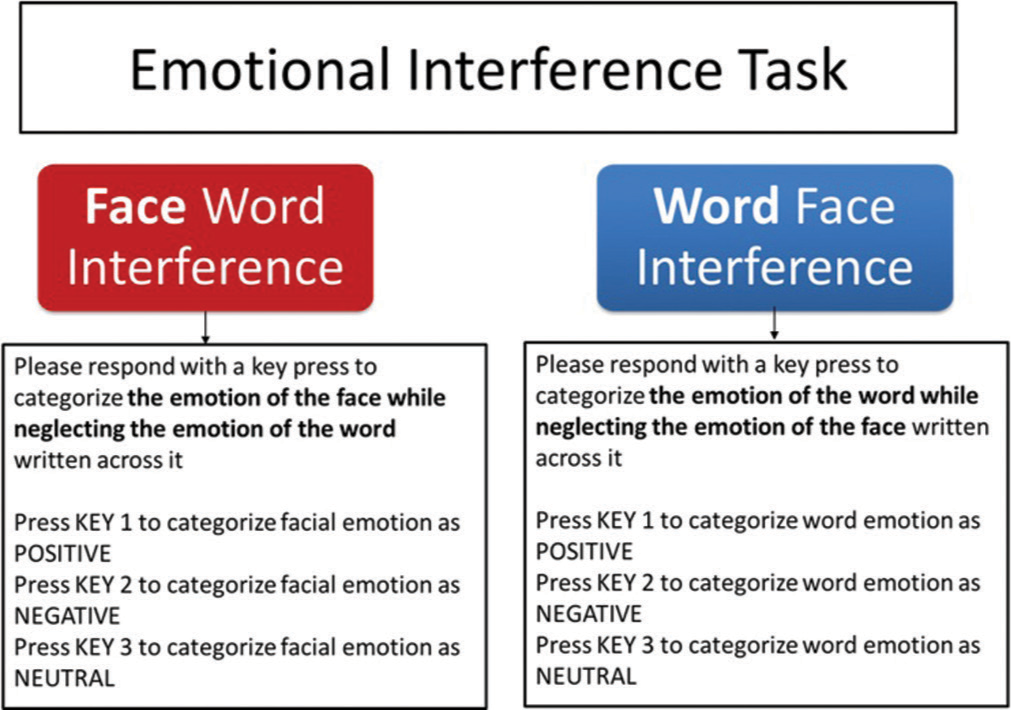
- The emotional interference task. Two variants of the task (Face word interference and Word face interference) were designed based on type of instruction and response.
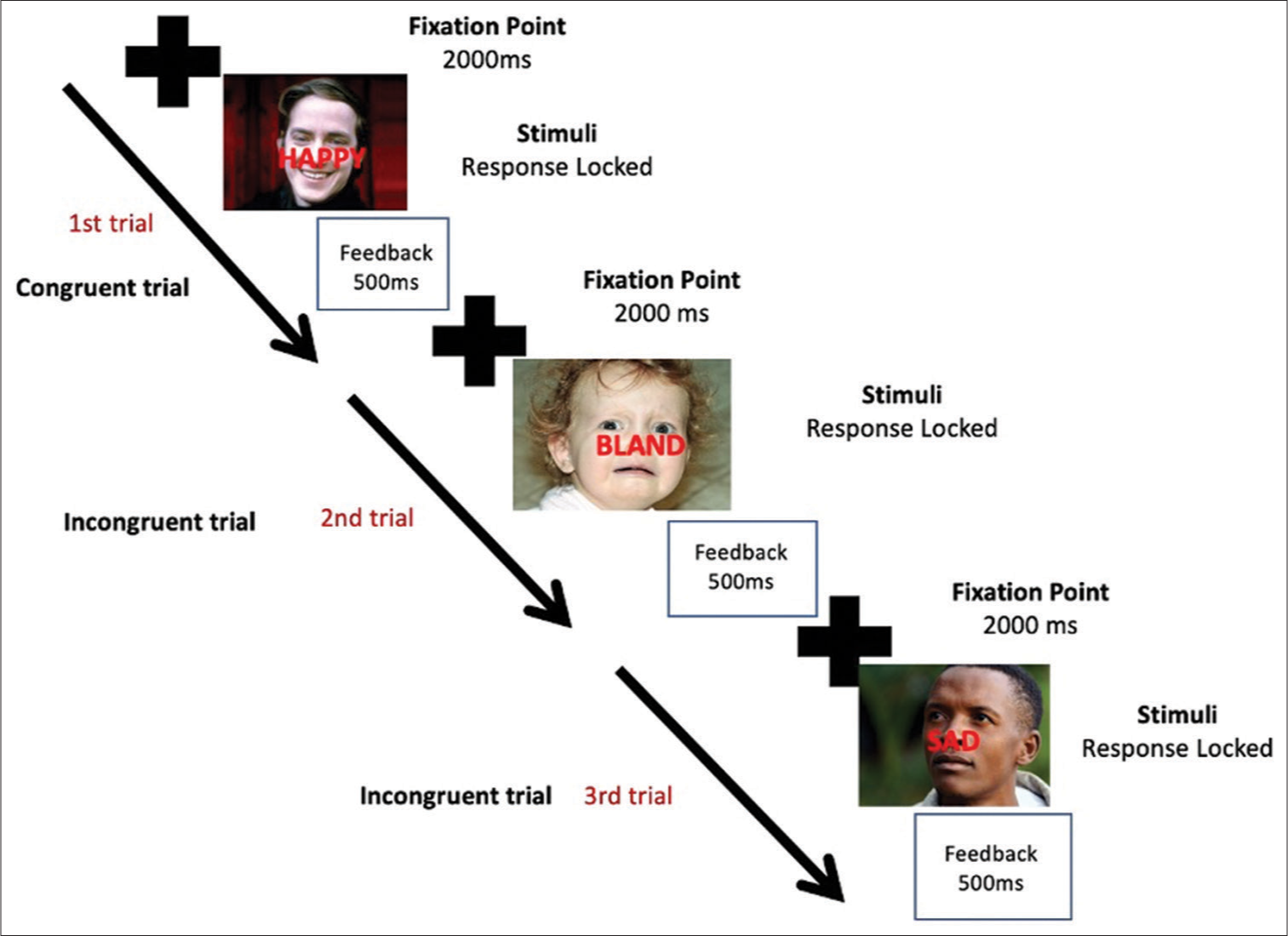
- Trial structure of emotional interference task. Fixation for 2000 ms and, Stimuli which was response locked and Feedback for 500 ms (accuracy percentage and reaction time) comprised of a single trial. The correct key press response will be dependent on the instruction during Face word Interference Task and Word face Interference task (details in text and Figure 1).
WfI
A WfI task (also Word face called emotional interference task) has been designed using Eprime V2. The instruction for the participant was to categorise the emotion of the word (target) overlaid on the faces shown by key press and ignore the emotional category of the face (distractor) underlying the word. [Figures 1 and 2] provide examples of stimuli and accompanying accurate responses during WfI task trials. When performing the WfI task, key 1 will be pressed in the first trial for the positive category of emotion of the word ‘HAPPY’ while disregarding the positive facial expression serving as a distractor. Key 3 should be pressed in the second trial for the neutral category of emotion of the word ‘BLAND’ while ignoring the negative facial emotion serving as a distractor and similarly, key 2 should be pressed for the negative category of emotion of the word ‘SAD’ in the third trial while disregarding the neutral facial emotion acting as a distractor.
Sixty trials consisting of 30 congruent (facial emotion and emotion of word have same category of emotion) and 30 incongruent (facial emotion and emotion of word belong to different category) trials were randomly presented during this study in two blocks as depicted in [Figure 2] (Batabyal et al., 2018). Simultaneous acquisition of EEG was recorded for both the tasks in all the subjects as described below.
Acquisition and pre-processing of EEG data
EEG data were acquired using 128-electrode Hydrocel Geodesic Sensor Net system (Philips Neuro, now MagStim USA). Common vertex (Cz) was used as recording reference and impedance was kept <50 kΩ. The raw data were band pass filtered into the 0.1–100 Hz bands online with a sampling frequency of 1000 Hz. Subsequently, offline preprocessing was done for artifact removal (eye movement, eye blink, cardiac and muscle artifacts) and replacement of the bad channels using Netstation 5.0.
EEG source analysis
The pre-processed EEG data were input to the Geosource of Netstation 5.2. The intracortical electrical sources were ascertained using standardised low-resolution electromagnetic tomography (sLORETA algorithm. The EEG trial segment of 1000 ms pre-stimulus till 2400 ms post stimulus was taken for the source analysis between FwI and WfI tasks [Figure 3]. Source activity at 2447 voxels located in 66 gyri was estimated using craniocerebral correlations based on the principle of inverse modelling.[15] Standardised MNI and Talairach stereotactic coordinates were used for the normalisation of cortical projections.[16]
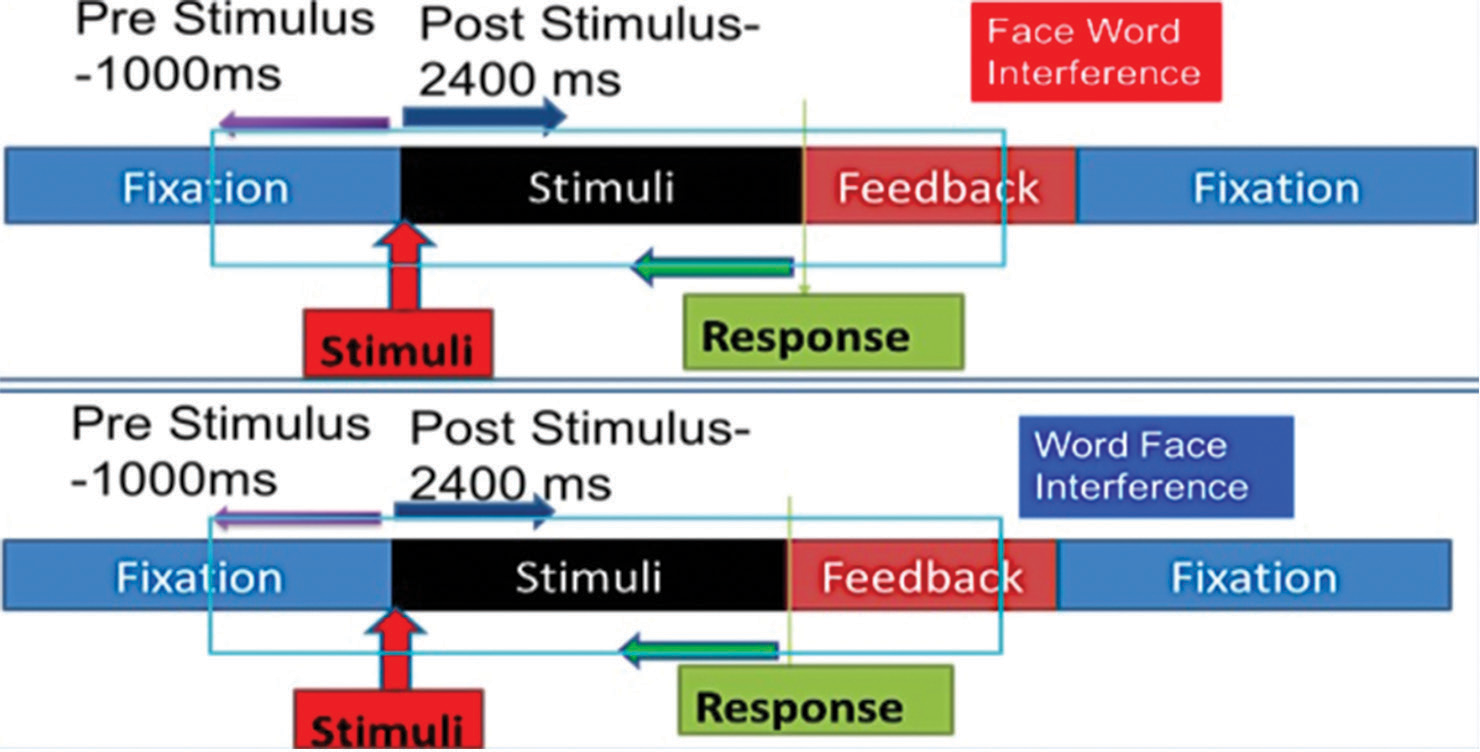
- Electroencephalogram (EEG) segmentation strategy for each emotional interference task trials. The block shows period of fixation, stimuli and feedback of a single trial. Each EEG segment comprised 11000 ms pre-stimulus and 2400 ms post-stimulus period.
Statistical analysis
Mean reaction time (RT) and accuracy percentage were computed for FwI, WfI, Congruent and Incongruent Trials of FwI and WfI respectively. Data were checked for normality as evidenced by P > 0.05 on the Kolmogorov–Smirnov goodness of fit test for normality. The mean current source density across 66 gyri were computed for 3400 ms epochs for 60 trials in both the tasks. Bonferroni correction was done by dividing the P-value (‘alpha’) with the number of hypotheses tested (i.e., 66) to avoid multiple comparison problems. The behavioural and cortical activity between FwI versus WfI was calculated using parametric and nonparametric paired test in MATLAB and SPSS.
RESULTS
The results are depicted as:
R1: Behavioural responses: Reaction time (RT) and accuracy percentage.
R2: Cortical sources as assessed by sLORETA using EEG.
R1: Behavioural
A Wilcoxon signed rank test and paired sample t-test were used for statistical group comparison. Results showed that subjects had significantly higher mean RT (t [19] = 8.165, P < 0.001, Cohen’s d = 1.689 and lower accuracy percentage [Z = −3.811, P < 0.001, with a large effect size r = 0.60 during the performance of FwI task compared to WfI task [Figures 4a and b]. Further on analysing congruent trials, higher RT and lower accuracy were seen in FwI compared to WfI (P < 0.001) [Figures 5a and b]. Similarly, separate analysis of incongruent trials also reflected higher RT and lower accuracy was seen in FwI compared to WfI (P < 0.001) [Figures 6a and b].
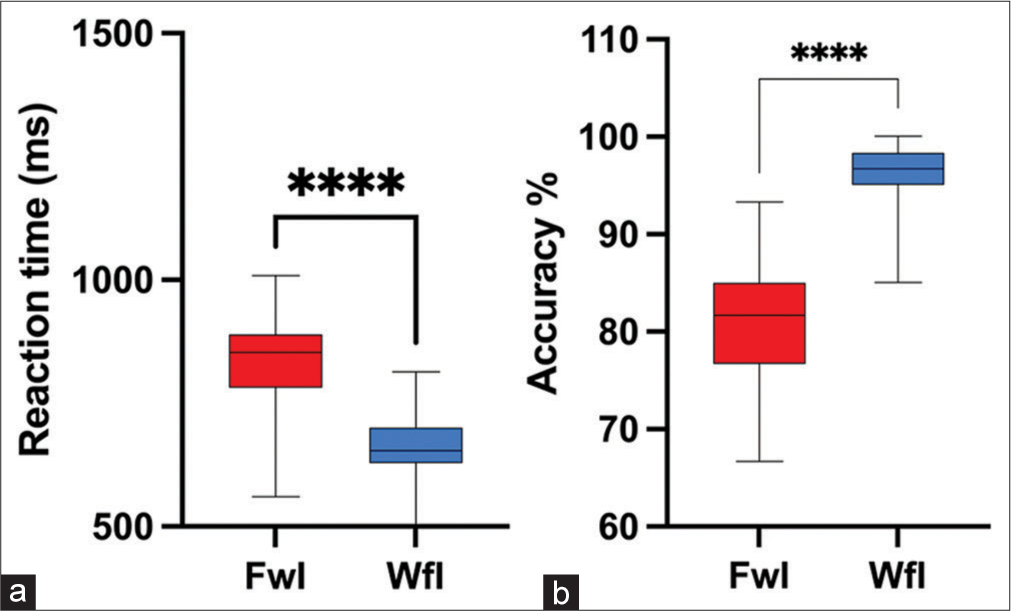
- Reaction time (a) and accuracy percentage (b) in Faceword interference (FwI) versus Wordface interference (WfI) task. ****: p<0.001.
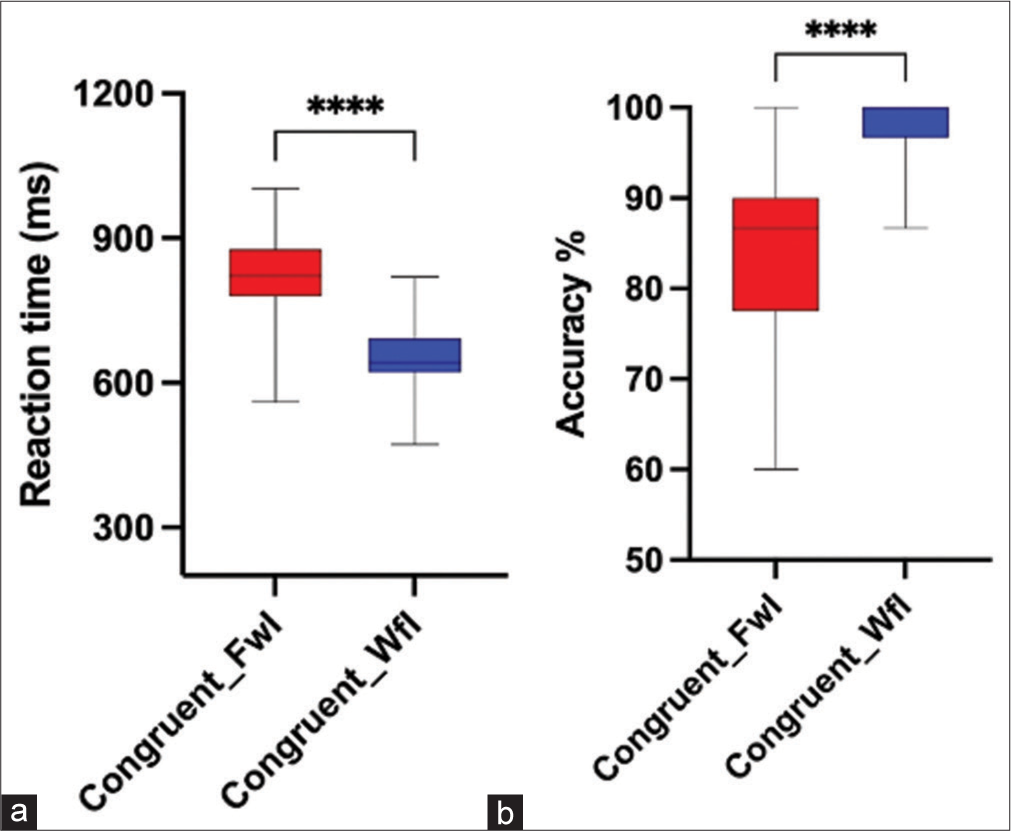
- Reaction time (a) and accuracy percentage (b) during congruent trials of Faceword interference (FwI) and Wordface interference (WfI). ****:p<0.001.
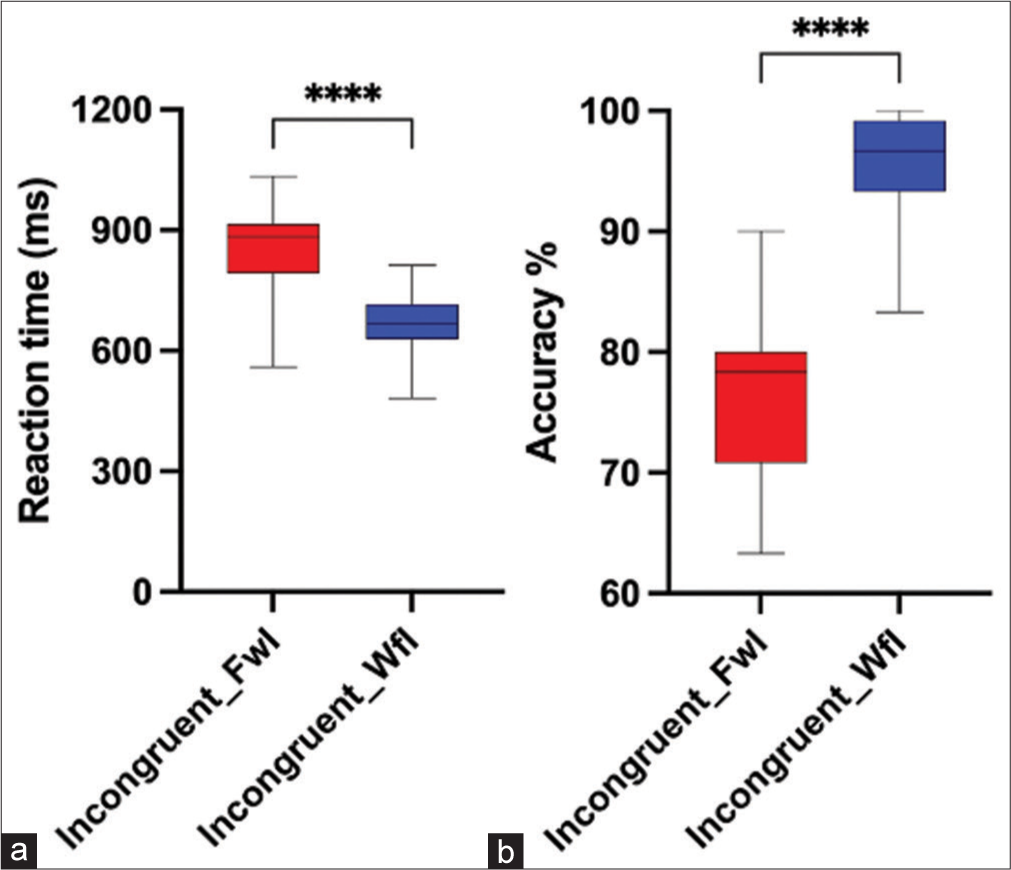
- Reaction time (a) and accuracy percentage (b) during incongruent trials of Faceword interference (FwI) and Wordface interference (WfI). ****:p<0.001
In addition, we observed that there was no significant difference in RT RT t (19) = −1.594, P = 0.127, Cohen’s d = 0.206, in incongruent trials of FwI as compared to congruent trials of FwI; however, incongruent trials of FwI showed significantly lower accuracy percentage (AccPerc) [Z]= −2.538, P = 0.011, with a moderate effect size r = 0.40 (Figures 7a and b as compared to congruent trials of FwI. Whereas, during WfI, significantly higher RT t (19) = −2.416, P = 0.026, Cohen’s d = 0.271 and significantly lower accuracy percentage (AccPerc) [Z]= −2.516, P = 0.012), with a moderate effect size r = 0.39 was observed during incongruent trials compared to congruent trials [Figures 8a and b].
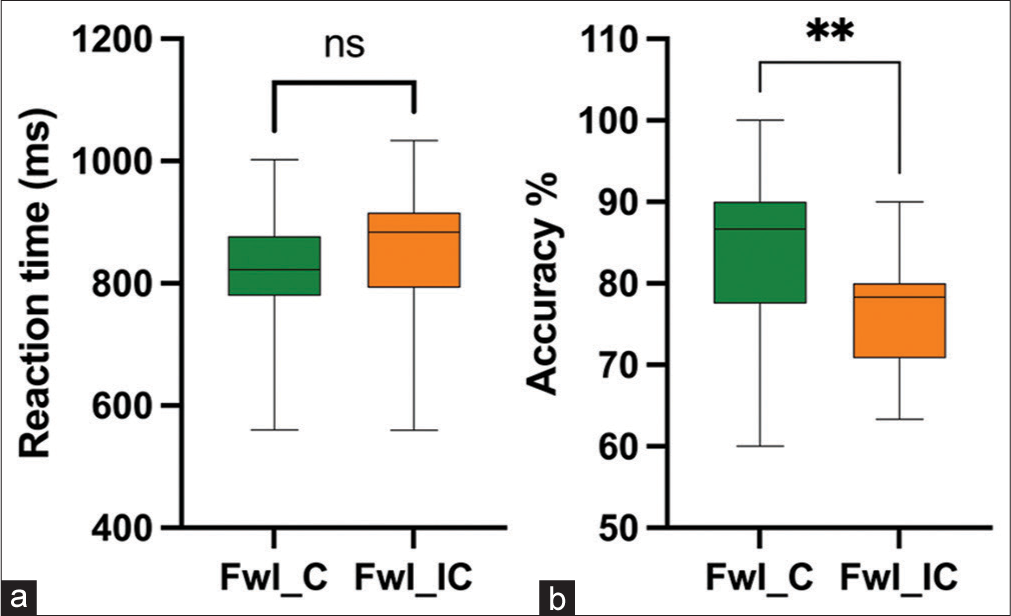
- Reaction time (a) and accuracy percentage (b) during congruent versus incongruent Faceword interference task. FwI_C: Face word Interference Congruent trials, FwI_IC: Face word Interference Incongruent trials, ns: Not significant, **: p=0.012.
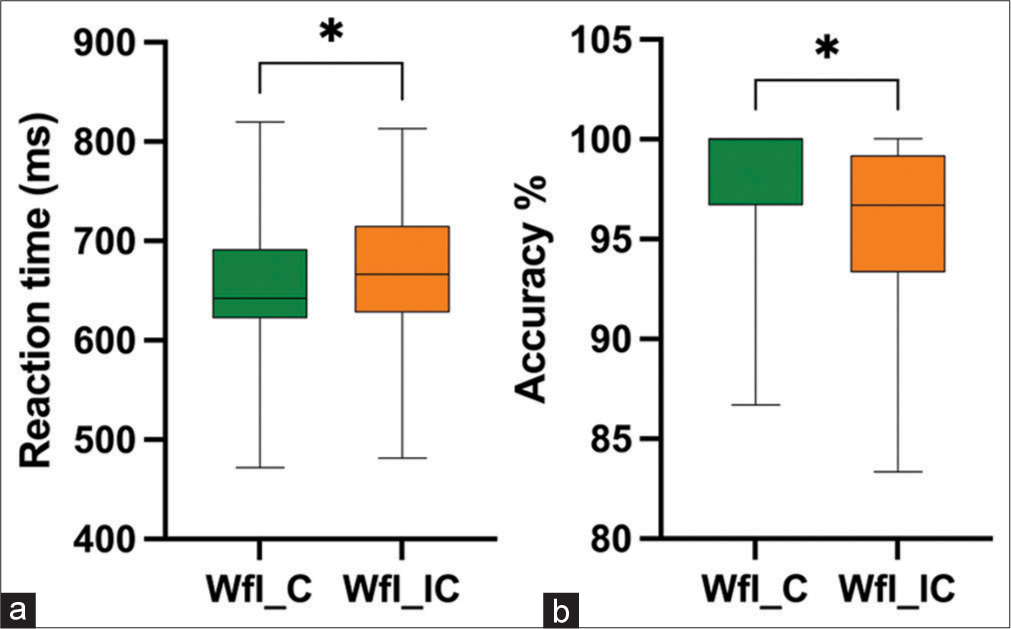
- Reaction time (a) and accuracy percentage (b) during congruent versus incongruent Wordface interference task. WfI_C: Word face Interference Congruent trials, WfI_IC: Word face Interference Incongruent trials, *: p<0.05.
R2: Cortical sources using EEG
Electrical source imaging was performed using sLORETA on Geosource toolbox of Netstation 5.3 with goal of illustrating all cortical areas associated with emotional interference task in which face and word have been used as a distractor. EEG trial segment of pre-stimulus 1000 ms till 2400 ms post-stimulus was taken for the source analysis [Figure 3]. This trial segment would reflect areas associated with emotion perception, attentional networks, cognitive control, executive function and motor areas.
Sixty-three areas showed significantly different activity between FwI and WfI. Out of them, higher cortical activity was seen in 60 areas, whereas significantly lower activity was seen in three in FwI condition [Figures 9 and 10].
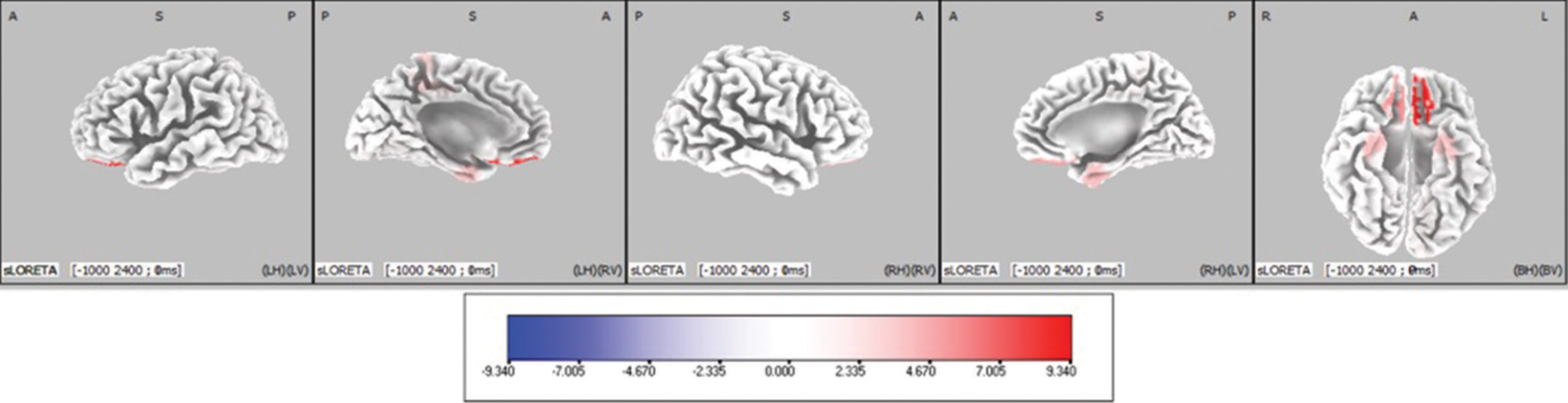
- Cortical areas showing significantly different activity between Face-word interference (FwI) and Word-face interference (WfI) have been marked in orthogonal plots. Blue coloured areas indicate decreased activity in Face word interference task as compared to Word face interference. The scale provided measures the t-statistics based on SnPM analysis comparing the current source density in (nA/mm2) between FwI and WfI.
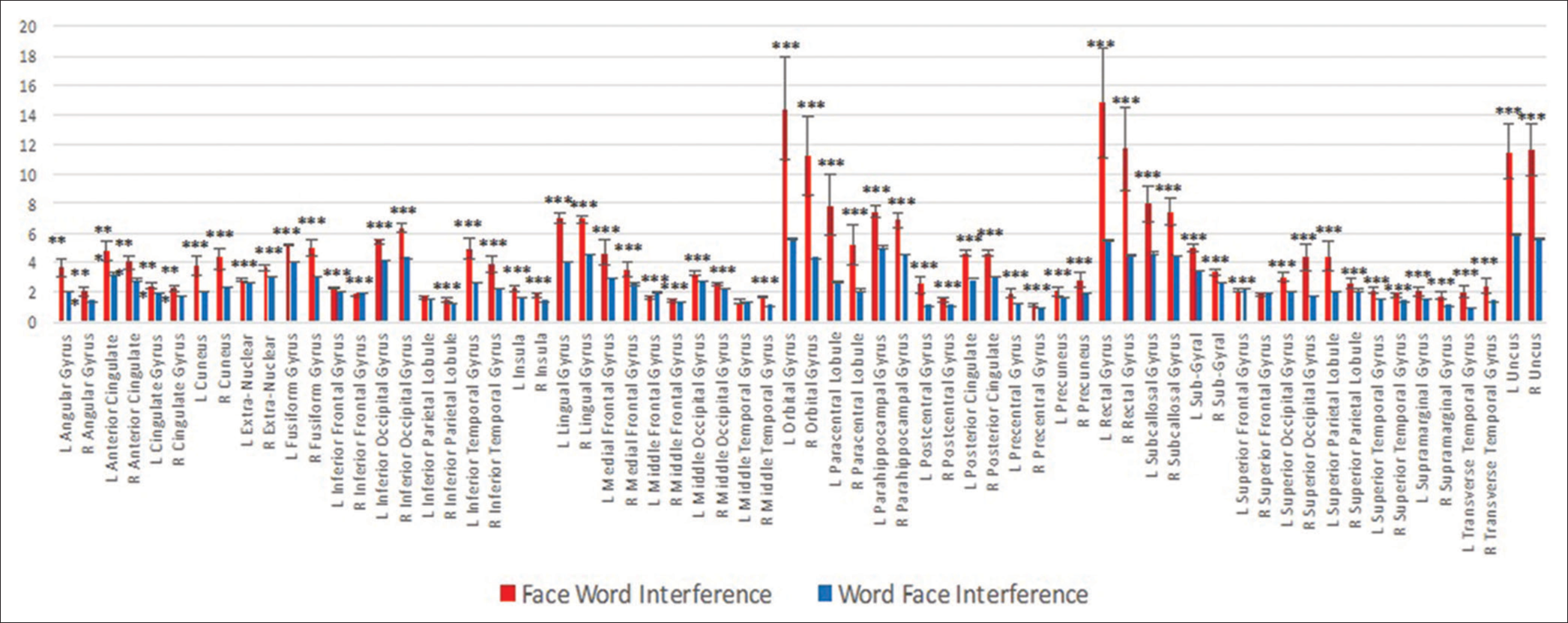
- Gyri showing significantly different activity have been compared between Face word and Word face interference. The significant areas (***P < 0.001) have been plotted.
DISCUSSION
The present study investigated emotional interference in healthy individuals using two emotional interference tasks that is, in FwI, (wherein subjects categorised facial emotion while word was distractor whereas in WfI (subjects categorised emotion of word while face was a distractor). RT and accuracy were ascertained during both the variants of emotional interference tasks and EEG data were acquired and analysed for underlying cortical sources.
We observed higher RT and lower accuracy percentage for categorising face in presence of word (FwI) versus categorising word in presence of face (WfI). This may be due to relatively automatic nature of word reading and distractive potential of face. Further, separate analysis of congruent and incongruent trials also revealed higher RT and lower accuracy percentage during FwI compared to WfI, thus substantiating the overall effect of the stimuli rather than the congruency/incongruency. The results are in line with Ovaysikia et al., (2011) wherein similar paradigm was used to assess emotional interference in healthy individuals.[11] We also observed higher RT and lower accuracy percentage in incongruent trials as compared to congruent trials during WfI task This indicates that faces act as robust distractors while reading words and the interfering facial emotional features underlying word, increases the processing time and chances of causing error to make a decision, while selectively attending to word emotion and ignoring facial emotion as a distractor. Further, to support the robustness of faces as distractors, we observe that RT is not significantly different between congruent and incongruent trials of FwI wherein faces were used as target stimuli and words as distractors. Thus, it indicates, that words do not cause significant interference when used as distractors in an emotional interference task. Contrary to this finding, Beall and Herbert in 2008, reported that words exhibit larger interference effects compared to facial expressions, thereby emphasising the automatic processing of the affective faces.[17] The probable explanation of difference with respect to our task may be due to the difference in stimuli structure in their tasks wherein the superimposed words had smaller font size with dark grey font colour; placed on the nose of the face. This might have caused easier identification of facial expressions resulting in words producing larger interference compared to faces.
In addition, we observed that accuracy percentage in incongruent trials were lower as compared to congruent trials in FWI, which implies that interference in presence of faces and words with different emotional valence can cause executive interference and result in response error.
To understand the cortical sources associated with emotional interference, source analysis was done on the EEG data acquired during both the tasks and cortical areas activity in terms of current source density were computed from 2447 dipoles representing 66 cortical gyri using sLORETA algorithm. We obtained significant different activity in 63 areas between FwI and WfI as shown in Graph 1, [Figure 5]. Out of those 63 areas, significantly higher activity was seen in 60 areas whereas significantly lower activity was seen in three areas during FwI task compared to WfI. This included medial prefrontal cortex and middle frontal gyri, which have been reported to be involved in pivotal behaviours (facial recognition or reading words) involving hierarchical processing.[11] The above-mentioned areas are also part of the saliency network and are concerned with unmasking of novelty in stimuli by merging of top-down inhibition of exogenous attention drawing circuits and endogenous task associated attentional pathways.[11] This explains that emotional faces by virtue of its saliency might draw attention, reflecting as higher activity during FwI.
Notably, enhanced activity of ACC and cingulate gyrus were also seen during FwI task. These areas have critical role in initiation, motivation and goal-directed behaviours. Further, cognitive domain of cingulate cortex is involved in response selection rather than affective domain, which is predominantly involved in autonomic reactivity.[18] On similar lines, several authors explored the functional aspect of ACC during regulation of emotional information[19,20] and while the emotional stimuli is delineated from the other relevant emotional distractors, as in our study paradigm.[21] Further, Ochsner et al. in 2004 explored the neural basis of affective and cognitive conflicts and documented key role of ACC in top-down control.[22] Hence, it is plausible cause for the higher activity in ACC during emotional interference tasks.
Interestingly, the areas involved in emotional processing (fusiform gyri, superior temporal gyri and occipital gyri) had higher activity during FwI.[23] The middle and superior occipital cortices are reported to be associated with word and face encoding, face-name association and emotional visual processing.[24,25] All the mentioned neural processes were predominantly associated with FwI task. Thus, the higher activity in these areas may be due to intermediate neural processing associated with perception and semantic association of face with its emotional categorisation.
In addition, higher activity was also observed in the right inferior parietal lobule. This could be due to its role in detection of saliency and increased alertness to emotional faces and words in FwI task.[26] The subjects’ engagement during the task may help to prioritise the given task instruction over exogenous stimuli (emotional faces and words).[27] The Insular cortex also exhibited higher cortical activity in our task. Insula has a role in processing of emotionally complex stimuli such as face, conflict monitoring, error awareness of task and goal-directed attention.[28] As expected, higher activity was also seen in parahippocampus gyri, which is known to be involved during the processing of faces.[29]
Lower cortical activity was seen only in three gyri, namely superior frontal gyri, middle frontal gyri and inferior, frontal gyri during FwI when compared to WFI. Lower activity of these areas has been previously reported in the literature during response inhibition tasks.[30] This can be the probable reason for decreased need of inhibiting tendency of the brain while attending to the word emotion during FwI and probably explain that emotional words are not as effective as emotional faces in drawing attention and disrupting performance at hand.[11]
Thus, the neural basis wherein face and words pose as a distractor involve the control of affective and cognitive conflicts as reflected by the areas involved during the performance of the emotional interference tasks.
The said study has potential limitations with respect to the limited sample size, due to which the effect of gender on emotional interference could not be studied in the present study. Future research can be done to look into the neural basis of incongruent versus congruent trials. Further, the effect of neutral stimuli can be studied using the similar paradigm with higher number of trials, which will strengthen the study envisaging the effect of cognition over emotion.
CONCLUSION
Evolutionarily reading faces have become an innate behaviour due to its survival relevance. Reading words is an automatic behaviour in adults by virtue of the learned nature associated with it. Hence, the cortical diaspora associated with either of them acting as a distractor is widespread. Higher cortical activity in areas associated with saliency, conflict and goal directed behaviour during FwI indicate the robustness of face processing and the hierarchical organisation of neural substrates in conditions of cognitive control. This study can further be extended in the patients with autism, attention deficit hyperactivity disorder (ADHD), addiction and other neuropsychiatric disorders, wherein in emotional interference is known to occur.
Significance statement
Emotional distractors affect the human cognitive control. Neural correlates of emotional interference can be studied using two emotional distractors (face/word) with the aid of 128 channel EEG. EEG source analysis revealed that the areas associated with saliency, conflict and goal directed behaviour have consistently higher activity in FwI compared to WfI indicating the robustness of face processing and the hierarchical organisation of neural substrates in conditions of cognitive control. This study can further be extended in the patients with Autism, ADHD, addiction and other neuropsychiatric disorders, wherein in emotional interference is known to occur.
Acknowledgments
The authors would like to thank the participants for the present study.
Ethical approval
The study has been approved by Institution’s Ethical Committee (Ref: IECPG-214/24.02.2016).
Availability of data and material
The authors have the data and relevant material of the said study.
Declaration of participant consent
Institutional Review Board (IRB) permission obtained for the study.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
All India Institute of Medical Sciences, New Delhi (Ref.F.8-456/A-456/2016/RS).
References
- Attention and control: Have we been asking the wrong questions? A critical review of twenty-five years In: Attention and Performance 14: Synergies in Experimental Psychology. Artificial Intelligence, and Cognitive Neuroscience. Cambridge, MA, US: The MIT Press; 1993. p. :183-218.
- [Google Scholar]
- Emotion and the motivational brain. Biol Psychol. 2010;84:437-50.
- [CrossRef] [PubMed] [Google Scholar]
- Learning where to look for danger: Integrating affective and spatial information. Psychol Sci. 2002;13:449-53.
- [CrossRef] [PubMed] [Google Scholar]
- Toward basic principles for emotional processing: What the fearful brain tells the robot In: Who Needs Emotions?: The Brain Meets the Robot. New York, US: Oxford University Press; 2005. p. :79-115.
- [CrossRef] [Google Scholar]
- Neural substrates of emotional interference: A quantitative EEG study. Neurosci Lett. 2018;685:1-6.
- [CrossRef] [PubMed] [Google Scholar]
- Half a century of research on the Stroop effect: An integrative review. Psychol Bull. 1991;109:163-203.
- [CrossRef] [PubMed] [Google Scholar]
- Neural basis of the emotional Stroop interference effect in major depression. Psychol Med. 2008;38:247-56.
- [CrossRef] [PubMed] [Google Scholar]
- Neural correlates of the emotional Stroop task in panic disorder patients: An event-related fMRI study. J Psychiatr Res. 2012;46:1627-34.
- [CrossRef] [PubMed] [Google Scholar]
- Microstates in resting-state EEG: Current status and future directions. Neurosci Biobehav Rev. 2015;49:105-13.
- [CrossRef] [PubMed] [Google Scholar]
- Word wins over face: Emotional Stroop effect activates the frontal cortical network. Front Hum Neurosci. 2011;4:234.
- [CrossRef] [PubMed] [Google Scholar]
- The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97-113.
- [CrossRef] [PubMed] [Google Scholar]
- Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion. 2001;1:276-98.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of color on emotions. J Exp Psychol Gen. 1994;123:394-409.
- [CrossRef] [PubMed] [Google Scholar]
- Automated cortical projection of EEG sensors: Anatomical correlation via the international 10-10 system. Neuroimage. 2009;46:64-72.
- [CrossRef] [PubMed] [Google Scholar]
- The problem of functional localization in the human brain. Nat Rev Neurosci. 2002;3:243-9.
- [CrossRef] [PubMed] [Google Scholar]
- The face wins: Stronger automatic processing of affect in facial expressions than words in a modified Stroop task. Cogn Emot. 2008;22:1613-42.
- [CrossRef] [Google Scholar]
- Contributions of anterior cingulate cortex to behaviour. Brain J Neurol. 1995;118(Pt 1):279-306.
- [CrossRef] [PubMed] [Google Scholar]
- Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871-82.
- [CrossRef] [PubMed] [Google Scholar]
- Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cereb Cortex. 2008;18:1475-84.
- [CrossRef] [PubMed] [Google Scholar]
- How brains beware: Neural mechanisms of emotional attention. Trends Cogn Sci. 2005;9:585-94.
- [CrossRef] [PubMed] [Google Scholar]
- For better or for worse: Neural systems supporting the cognitive down-and up-regulation of negative emotion. Neuroimage. 2004;23:483-99.
- [CrossRef] [PubMed] [Google Scholar]
- Neural substrates of facial emotion processing using fMRI. Brain Res Cogn Brain Res. 2001;11:213-26.
- [CrossRef] [PubMed] [Google Scholar]
- Differential activation in parahippocampal and prefrontal cortex during word and face encoding tasks. Neuroreport. 2001;12:2773-7.
- [CrossRef] [PubMed] [Google Scholar]
- Virtual needle pain stimuli activates cortical representation of emotions in normal volunteers. Neurosci Lett. 2008;439:7-12.
- [CrossRef] [PubMed] [Google Scholar]
- Visual attention circuitry in schizophrenia investigated with oddball event-related functional magnetic resonance imaging. Am J Psychiatry. 2007;164:442-9.
- [CrossRef] [PubMed] [Google Scholar]
- Changes in neuronal activation with increasing attention demand in healthy volunteers: An fMRI study. Synapse. 2001;42:266-72.
- [CrossRef] [PubMed] [Google Scholar]
- Common and unique components of response inhibition revealed by fMRI. Neuroimage. 2005;27:323-40.
- [CrossRef] [PubMed] [Google Scholar]
- Emotional responses to unpleasant music correlates with damage to the parahippocampal cortex. Brain J Neurol. 2006;129:2585-92.
- [CrossRef] [PubMed] [Google Scholar]
- Error-related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp. 2001;12:131-43.
- [CrossRef] [PubMed] [Google Scholar]







