Translate this page into:
Effect of Mentone® on depression- and anxiety-like profiles and regional brain neurochemistry in the adolescent Wistar Kyoto rat, a putative model of endogenous depression
*Corresponding author: Reshma A. Shetty, KSHEMA Centre for Genetic Services, K S Hegde Medical Academy, Nitte (Deemed to be University), Derlakatte 575 022, Mangaluru, Karnataka, India. drreshma@nitte.edu.in
-
Received: ,
Accepted: ,
How to cite this article: Shetty RA, Sadananda M. Effect of Mentone® on depression- and anxiety-like profiles and regional brain neurochemistry in the adolescent Wistar Kyoto rat, a putative model of endogenous depression. Indian J Physiol Pharmacol 2022;66:103-10.
Abstract
Objectives:
Antidepressants, when prescribed to treat adolescent depression tend to induce adverse effects, including suicidal tendencies. This is because the adolescent brain circuitry is still maturing and is therefore extremely vulnerable. As such, the search is on for compounds for use in complementary/alternative medicine. Polyherbal formulations are widely used as therapeutic alternatives for the treatment of depression. Such formulations and plant extracts are being studied in adult rodent models using standard pharmacological parameters, but not much emphasis has been given to testing the same in adolescents and endogenous animal models of depression. Therefore, the present study was focused on testing out the effect of the polyherbal formulation Mentone® on depression- and anxiety-like profiles and brain neurochemistry in the adolescent Wistar Kyoto rat (WKY), a putative model of endogenous and treatment-resistant depression (TRD).
Materials and Methods:
Mentone®, a polyherbal formulation comprising of four different plant species: Centella asiatica (Brahmi), Evolvulus alsinoides (Shankapushpi), Tinospora cordifolia (Guduchi), and Glycyrrhiza glabra (Yashtimadhu) was tested at two (18 and 36 mg/kg body weight) doses from the post-natal day (pnd) 25 to pnd 42 using standard neurobehavioral paradigms. Vehicular controls were intubated with saline and positive controls with 10 mg/kg body weight of conventional antidepressant, Fluoxetine. From pnd 35 onwards, animals were tested on a battery of tests, including sucrose preference, novel open field, elevated plus maze, and forced swim or Porsolt’s learned helplessness test. On pnd 42, animals were sacrificed and brain regional tissues such as the Prefrontal cortex (PFC), Striatum (Str), Nucleus Accumbens (NAc), and Hippocampus were microdissected out and subjected to reverse phase HPLC for the separation and quantification of monoamines: Norepinephrine (NE), dopamine (DA), serotonin (5-HT) and their metabolites, 3,4-Dihydroxyphenylacetic acid (DOPAC) and 5-hydroxyindoleacetic acid (5-HIAA) in reference to external standards.
Results:
Mentone® reversed anhedonia by increasing sucrose consumption in Mentone®-treated as compared to Fluoxetine-treated groups. However, there was no effect on anxiety-related parameters in the novel open field or elevated plus-maze. Mentone® exhibited significant anti-depressant-like effects as indicated by its ability to reduce swim stress-induced immobility in Porsolt’s behavioural despair test with a concomitant increase in climbing or struggling behaviour, signifying reversal of depressive-like symptomatology. HPLC-based separation and quantification of brain regional levels of monoamines and their metabolites revealed increased DA levels in NAc and Str in treated groups with decreased levels of metabolite DOPAC in Mentone®-treated groups indicating increased DA tone. Significantly reduced 5-HT metabolite 5-HIAA levels in both PFC and Str is indicative of increased 5-HT tone in both Mentone®- and Fluoxetine-treated groups. NE was variably affected.
Conclusion:
While no anxiolytic effects and differential neurochemical effects were observed in brain regional areas in relation to Mentone® and Fluoxetine treatment, anhedonia and forced swim test, which are gold-standard tests for assessing depressive-like profiles indicated an effect of Mentone® that was on par with Fluoxetine. Thus, studies on such Ayurvedic formulations would enable a teasing out or differentiation between anxiolytic-like and depressive-like symptomatology and could constitute a source that holds promise in the development of complementary/alternative therapies for the treatment of depression in general and TRD in particular.
Keywords
Depression
Anxiety
Antidepressants
Monoamines
Adolescence
Treatment-resistant depression
INTRODUCTION
Depression affects 3–5% of adolescents[1] worldwide with increasing projections in recent years. Adolescents show the same diagnostic criteria for depression as adults; however, sign indications may differ with developmental stage and few adolescents may show difficulty in identifying and describing internal mood states. Safe and effective treatment requires proper diagnosis and the use of therapies. In general, tricyclic antidepressants (TCAs) are not effective and may have adverse side effects. Evidence for the effectiveness of selective serotonin reuptake inhibitors (SSRIs) is limited. The third category is monoamine oxidase (MAO) inhibitors. All antidepressants carry the risk of suicidal tendencies in adolescents.[2]
Understanding of adult depression has come from studies using adult animals; therefore, studies using adolescent animals will help us to better understand adolescent depression. Recent studies have shown both neurochemical[2] and behavioural[3] differences between adult and adolescent animals after antidepressant treatment. The differences between adults and adolescents that occur not only in the response to antidepressants, but also in brain and behavioural development, as animal studies have shown, warrant a specific distinction between the study of adolescent and adult depression.
Very little is known as to how stress affects behaviour during puberty and how it develops in a sex-specific manner. Prescription of adult psychotic drugs to children and adolescents for these disorders is a case in point: Adolescents have been prescribed adult medications with undesirable side effects. Research for newer therapeutics with greater effectiveness and without or reduced adverse effects is needed. Natural antidepressant Bacopa monniera, a commonly used Ayurvedic drug has been reported to have significant anxiolytic activity when compared to anti-depressant imipramine.[4] Convolvulus pluricaulis, known commonly as Shankhpushpi, a psychostimulant, is known to improve memory and has been demonstrated to possess anxiolytic, antidepressant, and anti-stress like effects.[5] Root/rhizome extract of Nardostachys jatamansi has anti-depressant, anxiolytic, tranquilising, and psychotropic effects and has been used in the therapy of a wide variety of psychosomatic and mental disorders.[6]
Much research has been done in the sphere of ethnopharmacology by testing various natural compounds for potential anti-depressant activity.[7] Diverse preparations of plant extracts and readily available formulations have been studied in adult rodent models using standard pharmacological parameters.[4,5] However, not much emphasis has been given to testing the same in adolescent animals and animal models of endogenous depression. Moreover, long-term neurotrophic effects of the natural compounds have not been assayed.
One such polyherbal formulation is Mentone® which comprises components from four different plant species, namely, Centella asiatica (Brahmi), Evolvulus alsinoides (Shankapushpi), Tinospora cordifolia (Guduchi), and Glycyrrhiza glabra (Yashtimadhu). Some bioactive properties such as anti-microbial and anti-oxidant effects of the Mentone® formulation have been preliminarily assessed.[8,9] Bioactive properties of the individual plants have already been reported with emphasis on stress-related disorders.[10] Moreover, the synergistic effect of these plants has been proven in clinical trials for a proprietary license. Therefore, the effect of Mentone® as a potential anxiolytic or anti-depressant was tested out in adolescent Wistar Kyoto (WKY) rats.
Animal models offer researchers unique insights into the adolescent period. The WKY rat represents an endogenous model of depression[11,12] showing constancy with a classic childhood depressive profile[13] and exhibits abnormal behavioural patterns and abnormal neurochemistry.[11] The WKY was originally bred from the Wistar rat as a control strain for the spontaneously hypertensive rat, and also showed consistently exaggerated behavioural and physiological responses to stress[11,14] across a variety of situations in comparison to other strains, which shows its importance as an animal model of depressive-like behaviour[15] and anxiety vulnerability.[11,16] To measure the efficacy of antidepressants in reducing behavioural immobility during swim stress,[17] the forced swim test (FST) was used. It is one of the most commonly used tests to screen antidepressant activity and it is sensitive to all major classes of antidepressant drugs.[18,19]
A major proportion of patients fail to respond to treatment with antidepressant drugs, with treatment-resistant depression (TRD) representing a major component of the global burden of disease. Recently, a number of novel and experimental treatments have been introduced into clinical practice that appear to be effective for TRD.[20] The WKY model has recently[20] been suggested as a model for TRD. Therefore, the present study was undertaken to evaluate the effect of a putative Ayurveda-based therapeutic on adolescent WKY rats in relation to anxiolytic and hedonic responses, use the FST as a predictive validity measure in juvenile rats, and separate and quantify regional monoaminergic levels of brain areas implicated in depression such as Prefrontal cortex (PFC), Striatum (Str), Nucleus accumbens (NAc) and Hippocampus (HC).
MATERIALS AND METHODS
Subjects
Male WKY rats used in this study were obtained from ICMR-National Animal Resource Faculty for Biomedical Research, Hyderabad. Animals were housed in large cages and fed with standard food and water ad libitum. They were acclimated to standard environmental conditions of temperature (25–27°C), humidity, and 12 h light/dark cycles throughout the experimental period. Bodyweight and food intake were measured during the experiment. All experiments were conducted in the light cycle (9:00– 17:00 h) and accordance with the ethical regulations for animal experimentation laid by CPCSEA and cleared by the Institutional Animal Ethics Committee.
Experimental design
[Figure 1] shows the experimental design from weaning.

- Experimental design. pnd: Postnatal Day, SPT: Sucrose Preference Test, Hab: Habituation, OPF: Novel Open Field, EPM: Elevated Plus Maze, FST: Forced Swim Test, HPLC: High Pressure Liquid Chromatography.
Treatment
Rats were randomly divided into four groups. From postnatal day (pnd 25) onward Group I (C; n = 4) was treated with oral administration of saline (+Tween 20), Groups II (M-I; n = 4) and III (M-II; n = 4) with Mentone® at doses of 18 mg/kg and 36 mg/kg body weight, respectively, dissolved in 1 ml of vehicle, Group IV (F; n = 4) with standard drug Fluoxetine at a dose of 10 mg/kg bodyweight for 17 days, that is until mid-adolescence (pnd 42).
Behaviour
Behaviours of animals in the novel open field (OPF), elevated plus maze (EPM) and FST were recorded by a Panasonic CCD camera interphased with a frame grabber card and analysed by Ethovision 9.0® (Noldus, Netherlands). All the mazes were templated using Ethovision. Analysis was carried out blind. The accuracy of the Ethovision analysis was confirmed by manual analysis. Experiments were carried out under lighting conditions of 8.2-8.8 lux.
Anhedonia
The sucrose preference test was assessed for all the groups. Two bottles containing 1% sucrose solution and water available for 48 h (pnd 36-pnd 38). The locations of the bottles were rotated each 24 h periods. At the beginning and end of the final 48 h period both bottles were measured. Sucrose preference was defined as sucrose solution consumed/total liquid (sucrose + water) consumed × 100%.
Novel open field
On pnd 39 rats were exposed to OPF which was an open cube of 100 × 100 × 40 cm and behaviour recorded for five min. OPF was divided into inner and outer arenas. Dependent variables recorded were latency to leave the centre, time spent in the centre and periphery, total ambulation, number of rearings, and defecation.
Elevated plus maze
On pnd 39 rats were exposed to the EPM, which was carried out as described elsewhere in detail[21,22] with recording with Ethovision® 9.0 (Noldus, Netherlands). Anxiety index was calculated as open arm time and open arm entries in relation to total open arm time and total open arm entries as defined by Cohen et al.[23] Non-classical anxiety measures such as stretch-attend postures and nose dips were defined as per Melo et al.[24]
FST
To assess the antidepressant activity of Mentone®, a modified FST was conducted[25,26] which involved an initial 10-min training session (pre-test session) on pnd 40 followed 24 h later on pnd 41 by a test session for 5 min. It was carried out and measures taken as described in detail elsewhere.[21,22] All sessions were recorded by Ethovision software and analysis was carried out.
Brain monoamine and metabolite estimation
After the FST, rats were deeply anaesthetised, decapitated, brains quickly dissected out on the ice, and brain regions of interest (PFC, HC, Str, and NAc) punched out following the Paxinos and Watson Brain Atlas.[27] Tissues were cryopreserved, thawed and homogenised. Sample injection and separation and quantification of monoamines and their metabolites were carried out using a reverse-phase Waters HPLC system with electrochemical detection as described by us in detail elsewhere.[22]
Statistical analysis
All data are represented as Mean ± SEM. A one-way ANOVA was carried out to detect group differences. Bonferroni post hoc tests were carried out to check for significant differences between individual group means.
RESULTS
Overall, Mentone® induced a reversal of anhedonia, did not affect anxiety levels, reduced learned helplessness in the FST, and led to modulation of central monoamines and their metabolites that were comparable with conventional anti-depressant Fluoxetine. Dose-dependent effects in relation to vehicular Control and Fluoxetine-treated groups are mentioned in detail.
Sucrose preference test - Anhedonia
At both doses tested, Mentone® induced an increase in sucrose consumption and therefore reduced anhedonia [Figure 2]. post hoc analyses indicated a significant (P < 0.05) increase in the 36 mg/kg. b.w. group which was comparable to the Fluoxetine-treated group (F(3,20) = 7.468; P = 0.013).

- Sucrose preference test: Measure of anhedonia. Data are expressed as Mean ± SEM. *P < 0.05 indicates significant differences between treatment group versus control.
Novel open field - Exploratory behaviour and anxiety
One-way ANOVA did not reveal any significant differences between the groups in any of the tested parameters in the novel open field [Table 1 and Figure 3].
| Variables | Control | Mentone®-I | Mentone®-II | Fluoxetine | F statistic;Pvalue |
|---|---|---|---|---|---|
| Distance (m) | 21.34±0.47 | 18.20±1.30 | 16.65±1.91 | 17.55±2.06 | F(3,20)=1.170;P=0.358 |
| Rearing (no.) | 38.67±4.37 | 24.20±3.00 | 25.60±3.51 | 20.50±6.70 | F(3,20)=2.475;P=0.107 |
| Fecal boli (no.) | 4.33±0.33 | 3.00±1.00 | 4.00±1.52 | 2.66±1.45 | F(3,20)=0.453;P=0.722 |
| Entries to Centre (no.) | 19.67±5.92 | 13.20±3.18 | 10.20±2.81 | 9.00±1.78 | F(3,20)=1.647;P=0.227 |
Data are expressed as Mean±SEM.

- Anxiety-related parameters in novel open field: Bars represent Mean ± SEM of Centre (F(3,20)= 1.305; P = 0.314) and Periphery (F(3,20)= 1.305; P = 0.314) duration in Mentone®- and Fluoxetine-treated rats in relation to Vehicle-treated controls (C).
Elevated plus-maze - Anxiety
No differences were observed in any of the parameters, whether anxiety-related or exploration-related between treated groups and controls in the EPM [Table 2].
| Variables | Control | Mentone®-I | Mentone®-II | Fluoxetine |
|---|---|---|---|---|
| Duration% | ||||
| Open arm | 6.84±2.87 | 9.06±5.91 | 12.32±4.82 | 6.95±3.82 |
| Closed arm | 84.16±1.63 | 82.37±5.53 | 79.25±4.16 | 85.62±3.03 |
| Centre time | 8.08±1.12 | 42.60±14.89 | 38.50±9.79 | 9.16±1.76 |
| No. of Entries% | ||||
| Open arm | 24.33±6.32 | 35.63±8.82 | 37.62±4.89 | 31.50±5.89 |
| Closed arm | 75.67±6.32 | 64.37±8.82 | 62.38±4.89 | 68.50±5.89 |
| Anxiety index | 0.84±0.04 | 0.79±0.06 | 0.80±0.06 | 0.88±0.06 |
| Latency (s) | 29.27±18.95 | 64.27±56.65 | 6.52±2.81 | 34.50±29.63 |
| Distance (m) | 21.28±1.25 | 14.92±2.38 | 16.68±1.13 | 15.16±1.24 |
| Head dips | 4.00±1.53 | 4.20±1.88 | 5.40±2.36 | 5.25±2.36 |
| Rearings (no.) | 14.67±1.20 | 8.40±2.16 | 11.40±1.47 | 10.50±2.25 |
| SAP (no.) | 5.33±1.86 | 3.00±0.32 | 3.20±0.58 | 3.25±1.03 |
Data are represented as Mean±SEM. Animals were tested for 5 min.
FST - Behavioural Despair and Learned Helplessness
Mentone® induced a decrease in immobility in FST accompanied by an increase in climbing (struggling) behaviour at both doses (18 mg and 36 mg/kg b.w.) tested, which was comparable with conventional anti-depressant and SSRI, Fluoxetine (10 mg/kg b.w.).
Habituation – Behavioural despair
Mentone® at both concentrations of 18 and 36 mg/kg b.w. and Fluoxetine induced a decrease in immobility [Figure 4a] which was however, not significant (F(3,20) = 3.23; P > 0.05). Swimming behaviour (F(3,20) = 2.81; P > 0.05) was comparable across groups [Figure 4b], while climbing/struggling was increased in the treated groups [Figure 4c], though not significantly (F(3,20)= 1.668; P > 0.05). Time taken to become immobile (F(3,20) = 0.265; P > 0.05) was similar across groups [Figure 4d].
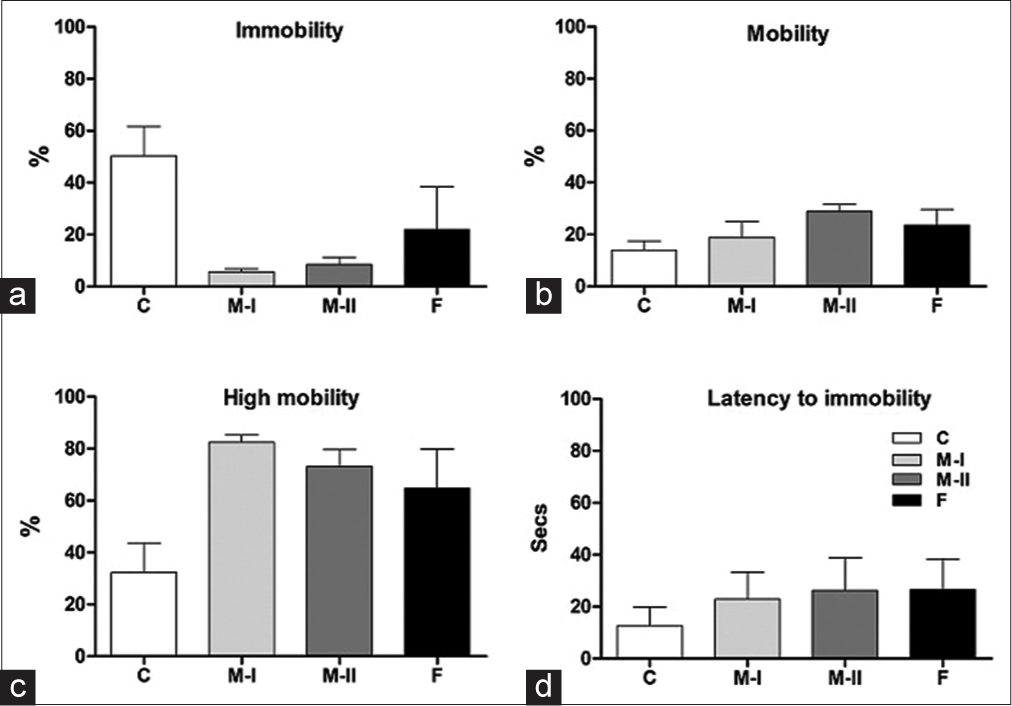
- (a-d) Immobility, mobility (swimming), high mobility (climbing) and latency behaviours of treated rats in the FSTHabituation. Data presented as Mean ± SEM.
Test – Learned helplessness
A significant effect of treatment on immobility (F(3,20) = 4.84; P < 0.05) and climbing/struggling behaviour (F(3,20)= 4.751; P < 0.05) was observed in the treated groups, whereas swimming (F(3,20) = 0.327; P > 0.05) and time to become immobile (F(3,20) = 0.247; P > 0.05) were not affected [Figures 5a-d]. Post hoc comparisons showed that rats treated with Mentone® at both doses tested, that is 18 and 36 mg/kg b.w., spent significantly (P < 0.05) lesser time immobile than controls and were comparable to Fluoxetine-treated rats. Rats treated with 36 mg/kg. b.w. Mentone® spent significantly more time climbing than Controls (P < 0.05), an effect that was similar to the lower dose of Mentone® and the Fluoxetine-treated groups.
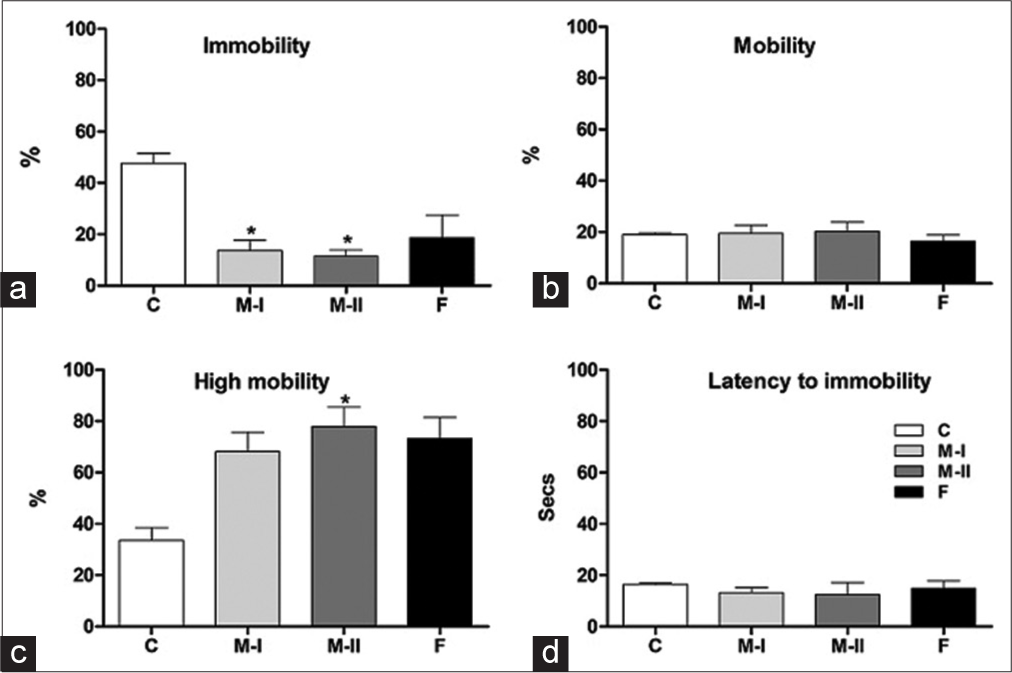
- (a-d) Immobility, swimming (mobility), climbing (high mobility) and latency behaviours of treated rats in the FST-test. Data presented as Mean ± SEM; P < 0.05.
Brain monoamines and metabolites
Mentone® and Fluoxetine treatment induced an increase in DA in NAc and Str, while it was not affected in PFC and HC [Figure 6a]. DOPAC was marginally decreased in PFC, Str, NAc, and HC in Mentone®-treated rats. Mentone® demonstrated variable effects on 5-HT levels, which were decreased in NAc when compared to Control and Fluoxetine-treated rats [Figure 6b]. NE was variably affected [Figure 6c]. 5-HIAA levels were marginally decreased in PFC, Str, and NAc in Mentone®-treated and not affected in HC [Table 3].
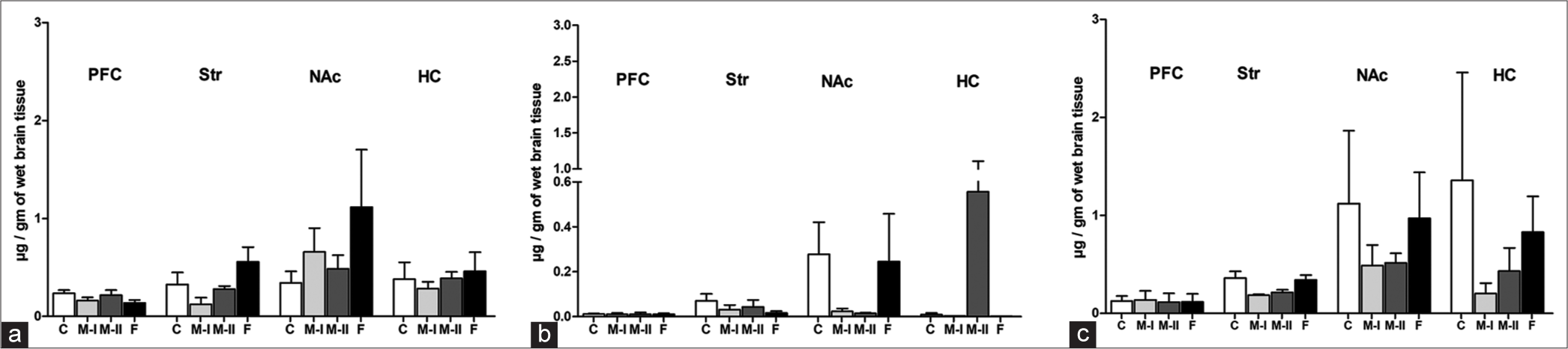
- Brain regional concentrations of Dopamine (a), Serotonin (b) and Norepinephrine (c) in Mentone®-I (M-I), Mentone®-II (M-II), Fluoxetine-treated (F) and Vehicular Control (C) rats in µg/g of wet tissue. Data are expressed as Mean ± SEM.
| Metabolite (µg/g) | Brain Regions | Controls | 18 mg Mentone® | 36 mg Mentone® | Fluoxetine | F-Statistic;P value |
|---|---|---|---|---|---|---|
| DOPAC | PFC | 0.050±0.012 | 0.020±0.001 | 0.023±0.010 | 0.083±0.038 | F(3,7)=1.27;P=0.36 |
| Str | 0.596±0.143 | 0.398±0.104 | 0.410±0.105 | 0.748±0.079 | F(3,7)=1.76;P=0.24 | |
| NAc | 2.018±1.063 | 0.380±0.114 | 0.694±0.216 | 3.957±2.406 | F(3,7)=1.16;P=0.39 | |
| HC | 0.079±0.037 | 0.013±0.006 | 0.059±0.006 | 0.019±0.007 | F(3,7)=1.41;P=0.34 | |
| HVA | PFC | 0.142±0.012 | 0.070±0.0005 | 0.164±0.022 | 0.222±0.042 | F(3,7)=4.43;P=0.06 |
| Str | 3.370±0.808 | 1.898±0.132 | 1.671±0.211 | 3.402±0.821 | F(3,7)=2.486;P=0.13 | |
| NAc | 1.960±0.511 | 3.098±1.168 | 1.744±0.286 | 4.415±2.321 | F(3,7)=0.848;P=0.51 | |
| HC | 0.403±0.173 | 0.190±0.043 | 0.193±0.020 | 0.154±0.047 | F(3,7)=1.482;P=0.29 | |
| 5-HIAA | PFC | 0.178±0.002 | 0.045±0.032 | 0.026±0.002 | 0.038±0.002 | F(3,7)=18.49;P=0.008 |
| Str | 0.824±0.106 | 0.436±0.012 | 0.187±0.005 | 0.507±0.065 | F(3,7)=17.55;P=0.009 | |
| NAc | 0.403±0.067 | 0.199±0.075 | 0.444±0.168 | 1.779±0.680 | F(3,7)=2.89;P=0.12 | |
| HC | 0.275±0.058 | 0.276±0.006 | 0.241±0.023 | 0.581±0.110 | F(3,7)=25.21;P=0.004 |
3,4-dihydroxyphenylacetic acid (DOPAC), Homovanillic acid (HVA), 5-hydroxyindole-3-acetic acid (5-HIAA) in different brain regions; PFC, Str, NAc and HC of Control, 18 mg/kg b.w. Mentone® 36 mg/kg b.w. Mentone® and Fluoxetine-treated rats. Concentrations are given in µg/gm of wet brain tissue. Data represented as Mean ± SEM.
DISCUSSION
The main aim of this study was to evaluate the antidepressant effect of Mentone® on anxiety- and depression-related measures and on the levels of monoamines and their metabolites. The results showed that anxiety-related parameters as measured in the OPF and EPM were unaffected. This could have been due to inter-individual variations in anxiety levels or the reported heterogeneity in the WKY rat strain itself, as shown by others.[28] Mentone®-treated rats demonstrated increased sucrose consumption to levels that were comparable with conventional Fluoxetine indicative of reduced anhedonia or increased hedonic behaviour, a reversal of the symptom of depression that codes for face validity, that is anhedonia.
The FST is the gold standard for eliciting a depressive-like profile that is assessed by measuring behavioural immobility, which is reflective of a state of despair, a phenotype that is normally reduced or reversed by the administration of antidepressants.[18,29] WKY rats treated with curcumin (i.p.) for 10 days demonstrated a reduction in immobility in FST in acute and chronic (10 days) treatment which persisted also a week later, though the effect did not persist when tested after 2 weeks.[30] Here, both doses of Mentone®, that is 18 and 36 mg/kg b.w. induced a decrease in immobility in FST which was accompanied by a concomitant increase in climbing behaviour that was comparable to that in Fluoxetine treatment.
The increased levels of DA and decreased levels of metabolite DOPAC indicating reduced DA metabolism in NAc and Str and more synaptic DA in Mentone®- and Fluoxetine-treated rats may have induced the increased climbing and thereby reduced immobility in the FST. All in all, considerable neurochemical changes have been shown to occur during adolescence, which needs to be taken into account while interpreting the results. 5-HIAA, the major metabolite of 5-HT was found to be decreased in cerebrospinal fluid of Fluoxetine-treated patients with major depression.[31] Here, 5-HIAA was reduced in PFC and Str of both Mentone®- and Fluoxetine-treated groups. NAc also demonstrated a decrease in 5-HIAA levels in the Mentone®-treated group.
The previous studies using Mentone® showed that it attenuated distress systems and enhanced cognitive processes in humans.[32] Mentone is made up of the C. asiatica plant, the stem of T. cordifolia, and roots of E. alsinoides and G. glabra. C. asiatica is known to act on the nervous system by enhancing memory retention.[33] E. alsionoides have been reported in the treatment of neurodegenerative disorders,[34] while T. cordifolia and G. glabra have been found to be effective in the regulation of monoamines and have been shown to increase NE and DA, respectively.[35] T. cordifolia demonstrates presence of phenols, saponins, tannins glycosides, steroids, terpenoids and alkaloids and shows anti-stress activity.[36-43] C. asiatica has shown presence of triterpenes, alkaloids, phenolics, flavonoids, tannins terpenoids, saponins, steroids and glycosides, has been shown to have a role in the treatment of brain-related disorders and is known as a memory enhancer.[44-49] A synergistic effect of all these plants may have contributed to the anti-depressant effects demonstrated by Mentone® in this study.
While in the East, herbal compounds are part of traditional or Ayurvedic medicine, the West is only now waking up to these possibilities – St. John’s wort being prescribed for adolescent depressants is a case in point. Antidepressants, whether TCAs, SSRIs, or MAO inhibitors all have adverse effects with long-term use, which makes the search for natural compounds with no or lesser side effects all the more urgent. Further, though anxiety and depression are often said and found to be comorbid, our results indicate that teasing out the symptoms is possible. With the WKY being suggested as a model of TRD, implications of the results obtained here indicate the use of alternate/complementary therapies in targeted, symptom-based therapeutic strategies in adolescent depression.
CONCLUSION
Ayurveda is one of the oldest medicinal systems which provides therapeutically useful compounds and formulations. Therefore, such traditional knowledge can be leveraged by isolating, characterising, and empirically testing bioactive components from potential herbal sources that hold promise as better antidepressant drugs with fewer side effects.
Acknowledgments
Grant support from the Department of Biotechnology, Govt. of India [Grant No. BT/PR4676/MED/30/735/2012]; and the Department of Science and Technology, Govt. of India [Grant No. CSI/05/2009] to MS is acknowledged.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
The Department of Science and Technology, Govt. of India [Grant No. CSI/05/2009]. The Department of Biotechnology, Govt. of India [Grant No. BT/PR4676/MED/30/735/2012].
Conflicts of interest
There are no conflicts of interest.
References
- Depression: An evolutionarily conserved mechanism to terminate separation distress? A review of aminergic, peptidergic, and neural network perspectives. Neuropsychoanalysis. 2009;11:7-51.
- [CrossRef] [Google Scholar]
- Differential effects of psychoactive drugs in adolescents and adults. Crit Rev Neurobiol. 2005;17:51-67.
- [CrossRef] [PubMed] [Google Scholar]
- Differential behavioural and neurochemical outcomes from chronic paroxetine treatment in adolescent and adult rats: A model of adverse antidepressant effects in human adolescents? Int J Neuropsychopharmacol. 2011;14:491-504.
- [CrossRef] [PubMed] [Google Scholar]
- Antidepressant activity of standardized extract of Bacopa monnieri in experimental models of depression in rats. Phytomedicine. 2002;9:207-11.
- [CrossRef] [PubMed] [Google Scholar]
- Review on ethnomedicinal uses and phytopharmacology of memory boosting herb “Convolvulus pluricaulis” Choisy. Aust J Med Herb. 2010;22:19-25.
- [Google Scholar]
- Inhibition of MAO and GABA: Probable mechanisms for antidepressant-like activity of Nardostachys jatamansi DC. in mice. Indian J Exp Biol. 2008;46:212-8.
- [Google Scholar]
- The status and scope of Indian medicinal plants acting on central nervous system. Indian J Pharmacol. 1997;29:340.
- [Google Scholar]
- Anti-oxidant, Anti-inflammatory and Anti-microbial Properties of a Putative Nootropic Poly-herbal Formulation, Master's Dissertation Mangalagangothri: Mangalore University; 2016.
- [Google Scholar]
- Phyto-chemical Characterization and Antimicrobial Activity of a Putative Nootropic Poly-herbal Formulation, Master's Dissertation Mangalagangothri: Mangalore University; 2016.
- [Google Scholar]
- In vitro screening for anti-cholinesterase and antioxidant activity of methanolic extracts of ayurvedic medicinal plants used for cognitive disorders. PLoS One. 2014;9:e86804.
- [CrossRef] [PubMed] [Google Scholar]
- Animal models of anxiety vulnerability the Wistar Kyoto rat In: Selek S, ed. Different Views of Anxiety Disorders, Intech Open. 2011.
- [CrossRef] [Google Scholar]
- Selectively bred Wistar-Kyoto rats: An animal model of depression and hyper-responsiveness to antidepressants. Mol Psychiatry. 2003;8:925-32.
- [CrossRef] [PubMed] [Google Scholar]
- Two different putative genetic animal models of childhood depression a review. Prog Neurobiol. 2009;88:153-69.
- [CrossRef] [PubMed] [Google Scholar]
- Strain differences in the behavioral effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharmacology. 2000;22:191-9.
- [CrossRef] [Google Scholar]
- Behavioral effects of acute and chronic fluoxetine in Wistar-Kyoto rats. Physiol Behav. 1999;67:315-20.
- [CrossRef] [Google Scholar]
- Wistar-Kyoto rats as an animal model of anxiety vulnerability: Support for a hypervigilance hypothesis. Behav Brain Res. 2009;204:162-8.
- [CrossRef] [PubMed] [Google Scholar]
- Depression: A new animal model sensitive to antidepressant treatments. Nature. 1977;266:730-2.
- [CrossRef] [PubMed] [Google Scholar]
- Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology (Berl). 1988;94:147-60.
- [CrossRef] [PubMed] [Google Scholar]
- Antidepressant behavioral effects by dual inhibition of monoamine reuptake in the rat forced swimming test. Psychopharmacology (Berl). 1998;136:190-7.
- [CrossRef] [PubMed] [Google Scholar]
- The Wistar-Kyoto rat model of endogenous depression: A tool for exploring treatment resistance with an urgent need to focus on sex differences. Prog Neuropsychopharmacol Biol Psychiatry. 2020;101:109908.
- [CrossRef] [PubMed] [Google Scholar]
- Anxiety-and depressive-like profiles during early-and mid-adolescence in the female Wistar Kyoto rat. Int J Dev Neurosci. 2017;56:18-26.
- [CrossRef] [PubMed] [Google Scholar]
- Immediate and delayed anxiety-and depression-like profiles in the adolescent Wistar-Kyoto rat model of endogenous depression following postweaning social isolation. Behav Brain Res. 2017;320:323-32.
- [CrossRef] [PubMed] [Google Scholar]
- Early post-stressor intervention with high-dose corticosterone attenuates posttraumatic stress response in an animal model of posttraumatic stress disorder. Biol Psychiatry. 2008;64:708-17.
- [CrossRef] [PubMed] [Google Scholar]
- Antidepressants differentially modify the extinction of an aversive memory task in female rats. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37:33-40.
- [CrossRef] [PubMed] [Google Scholar]
- Behavioural despair in rats: A new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379-91.
- [CrossRef] [Google Scholar]
- Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl). 1995;121:66-72.
- [CrossRef] [PubMed] [Google Scholar]
- Sex differences in depressive, anxious behaviors and hippocampal transcript levels in a genetic rat model. Genes Brain Behav. 2013;12:695-704.
- [CrossRef] [PubMed] [Google Scholar]
- Assessing antidepressant activity in rodents: Recent developments and future needs. Trends Pharmacol Sci. 2002;23:238-45.
- [CrossRef] [Google Scholar]
- Antidepressant-like effects of curcumin in WKY rat model of depression is associated with an increase in hippocampal BDNF. Behav Brain Res. 2013;239:27-30.
- [CrossRef] [PubMed] [Google Scholar]
- A Critical Study on the Concept of Medha and Effect of Medhya Rasayana on Anxiety Levels of Exam Facing Students. MD Ayurveda Thesis, Karnataka University.
- [Google Scholar]
- Centella asiatica (Linn) induced behavioural changes during growth spurt period in neonatal rats. Neuroanatomy. 2005;4:18-23.
- [Google Scholar]
- An update on Shankhpushpi, a cognition-boosting Ayurvedic medicine. Zhong Xi Yi Jie He Xue Bao. 2009;7:1001-22.
- [CrossRef] [Google Scholar]
- In vitro qualitative and quantitative phytochemical analysis of ethanolic and 50% ethanolic extracts of Tinospora cordifolia, Momordica charantia, Cucurbita maxima and Raphanus sativus. Int J Pharm Sci Res. 2014;5:1937-41.
- [Google Scholar]
- Neuroprotective effect of curcumin in arsenic-induced neurotoxicity in rats. Neurotoxicology. 2010;31:533-9.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of quality of Tinospora cordifolia (willd.) Mier (Menispermacea): Pharmacognostical and phytophysicochemical profile. Pharm Glob. 2010;5:1-4.
- [Google Scholar]
- Chemistry and medicinal properties of Tinospora cordifolia (Guduchi) Indian J Pharmacol. 2003;35:83-91.
- [Google Scholar]
- Phytochemical screening and aphrodisiac property of Tinospora cordifolia. Studies. 2011;8:9.
- [Google Scholar]
- Screening of antibacterial potentials of some medicinal plants from Melghat forest in India. Afr J Tradit Complement Altern Med. 2009;6:228-32.
- [CrossRef] [PubMed] [Google Scholar]
- Antibacterial activity of Tinospora cordifolia (Willd) Hook. F. Thoms on urinary tract pathogens. Int J Curr Microbiol Appl Sci. 2013;2:190-4.
- [Google Scholar]
- Evaluation of methanolic extracts of in vitro grown Tinospora cordifolia (willd) for antibacterial activities. Asian J Pharm Clin Res. 2012;5:172-5.
- [Google Scholar]
- Chemical components of Centella asiatica and their bioactivities. Zhong Xi Yi Jie He Xue Bao. 2007;5:348-51.
- [CrossRef] [PubMed] [Google Scholar]
- A new triterpene and a saponin from Centella asiatica. Chin Chem Lett. 2007;18:62-4.
- [CrossRef] [Google Scholar]
- Antimicrobial and antifungal activity of Centella asiatica (L.) Urban, Umbeliferae. Res J Pharm Technol. 2009;2:328-30.
- [Google Scholar]
- In vitro screening of five local medicinal plants for antibacterial activity using disc diffusion method. Trop Biomed. 2005;22:165-70.
- [Google Scholar]
- Antioxidative activity and total phenolic compounds of leaf, root and petiole of four accessions of Centella asiatica (L.) Urban. Food Chem. 2003;81:575-81.
- [CrossRef] [Google Scholar]







