Translate this page into:
Efficacy and safety of Saroglitazar and Fenofibrate in the treatment of diabetic dyslipidaemia: A pilot study
*Corresponding author: Bhupinder Singh Kalra, Department of Pharmacology, Maulana Azad Medical College, Delhi, India. drbskalra@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Gahlot R, Kumar S, Garg S, Chawla S, Kalra BS. Efficacy and safety of Saroglitazar and Fenofibrate in the treatment of diabetic dyslipidaemia: A pilot study. Indian J Physiol Pharmacol 2023;67:15-20.
Abstract
Objectives:
Diabetic dyslipidaemia (DD) is characterised by hypertriglyceridaemia and elevated or normal levels of low-density lipoprotein cholesterol and decreased levels of high-density lipoprotein cholesterol with Type 2 diabetes mellitus. Statins and anti-diabetic medication are coprescribed for optimal control.
Materials and Methods:
The objective of the study was to compare the safety and efficacy of Saroglitazar 4-mg and Fenofibrate 200 mg in combination with low dose Atorvastatin (10 mg) in patients with DD. Run-in period of 4 weeks for life-style and diet modification followed by 12 weeks of treatment with saroglitazar or fenofibrate and low dose of atorvastatin was followed. Primary outcome of this study was an absolute change in serum triglyceride level at baseline and end of treatment period (12 weeks). Secondary outcome was changed from baseline lipid profile, fasting blood glucose and glycosylated haemoglobin (HbA1c) at the end of treatment period. Safety assessment was also done during the duration of study.
Results:
Forty patients of DD were randomly divided into two groups. One group received Saroglitazar 4 mg along with Atorvastatin 10 mg. Patients in second group received Fenofibrate 200 mg along with Atorvastatin 10 mg. Improvement in deranged lipid levels in both the groups was observed and this difference in improvement statistically was not found to be significant. We also observed that Saroglitazar significantly improves glycaemic profile by decreasing fasting blood sugar levels and HbA1c (P = 0.01, P < 0.01). Adverse events reported during this study were mild and none of the patients reported serious adverse events.
Conclusion:
Saroglitazar could be a potential drug to control both hyperglycaemia and dyslipidaemia in patients with DD.
Keywords
Diabetic dyslipidaemia
Dyslipidemia
Fenofibrate
Hyperlipidaemia
Peroxisome proliferator-activated receptor
Saroglitazar
Triglycerides
INTRODUCTION
Diabetes mellitus (DM) is one of the most common chronic diseases globally responsible for increased morbidity and mortality. The global prevalence of diabetes among adults was estimated to be 6.4%, affecting 285 million people in 2010 and is expected to increase to 7.7%, affecting 439 million people by 2030. Between 2010 and 2030, it is estimated that there will be a 69% and 20% increase in number of adults with diabetes in developing countries and developed countries, respectively. Diabetes has evolved into an epidemic in India. The estimated number of patients with diabetes in India was 62.4 million in 2011 which is projected to rise to a staggering 101.2 million by 2030.[1]
Patients with Type 2 DM (T2DM) are associated with a considerably increased risk of premature atherosclerosis, particularly coronary heart disease and peripheral arterial disease, which is the leading cause of death in these individuals.[2] Epidemiologic studies have demonstrated that DM is an independent risk factor for cardiovascular disease.3 The mortality associated with a coronary event in people with DM is significantly higher than the mortality in non-diabetic individuals.[3]
Patients with diabetic dyslipidaemia (DD) invariably have mild to marked elevation of triglyceride (TG) rich lipoproteins – very low density lipoprotein-cholesterol (VLDL-C) and VLDL-C remnants and low levels of high density lipoprotein-cholesterol (HDL-C). In some patients with Type 2 DM, dyslipidaemia may be a consequence of the deranged metabolic state that is, hyperglycaemia and insulin resistance. In these patients, good control of hyperglycaemia might mitigate the dyslipidaemia.[4] However, since optimal glycaemic control generally is not possible in most patients with Type 2 DM, some degree of dyslipidaemia often persists regardless of attempts to normalise plasma glucose levels. Even after effective control of hyperglycaemia has been achieved, insulin resistance may not improve and may contribute to dyslipidaemia.
Diabetes-related changes in plasma lipid levels are among the key factors that are amenable to intervention. Existing therapy for the management of hypertriglyceridemia is lifestyle changes and pharmacotherapy with statins alone or in combination with fibrates, niacin, omega-3 fatty acids, ezetimibe, etc.[5] At high doses, statins may cause adverse effects in the form of myopathy or rhabdomyolysis, drug-induced eruptions, etc. Statin toxicity is usually observed as myalgia or muscle weakness with creatine kinase levels >10 times the normal upper limit. Rhabdomyolysis is the most severe adverse effect of statins, which may result in acute renal failure, disseminated intravascular coagulation and death. Hepatic function is also known to be affected by statin use.[6]
Recent data suggest that statin therapy for long-term, especially in high doses, can worsen the glycaemic control and can lead to new onset of T2DM.[7] Fibrates can also cause myopathy, occurring at a rate similar to that of statin therapy. The risk for myopathy appears to be elevated in patients with combination therapy with in patients with pre-existing renal dysfunction. This combination therapy leads to increased risk of myopathy. Fibrates should be avoided in populations with severe renal impairment.[8]
Saroglitazar has dual property of optimising levels of deranged TGs primarily and hyperglycaemia. Saroglitazar is a dual peroxisome proliferator-activated receptor (PPAR)-α/γ agonist. It has strong PPAR- α action with moderate PPAR-γ action.[9] Myopathy as an adverse effect is not documented with Saroglitazar. In trials with Saroglitazar, minor adverse effects such as gastritis, asthenia and dizziness have been reported. It is approved for the treatment of DD as add on therapy with statins. Therefore, this present study was designed to evaluate the safety and efficacy of saroglitazar and fenofibrate with low dose atorvastatin in T2DM patients having hypertriglyceridaemia.
MATERIALS AND METHODS
Study design
The study was a randomised, open label, parallel, single-centre, intervention and prospective study carried out for a duration of 12 months, from February 2019 to February 2020. The study was initiated after obtaining the approval from the Institutional Ethics Committee. The study was registered with Clinical Trial Registry of India (CTRI/2019/02/017410). The study was conducted in Department of Medicine and Pharmacology, Maulana Azad Medical College and Associated Lok Nayak Hospital, Delhi. During the 4-week run-in period, newly diagnosed cases of dyslipidaemia with a history of diabetes were put on dietary and life-style modification programmes.
Study population
After the 4-week run-in period, subjects were required to meet the inclusion criteria: (1) Diagnosed patients of DD with fasting TG >200–400 mg/dL, (2) patients in the age group of 18–65 years, (3) patients with a history of T2DM (glycosylated haemoglobin [HbA1c] >7–9%), (4) patients being treated with either a sulphonylurea, metformin for diabetes, or both treatments for at least 3 months and (5) patients in whom dyslipidaemia (TG level >200 mg/dL) is not controlled after 4 weeks of prescription with Atorvastatin 10 mg.
Exclusion criteria for this study were (1) patients on insulin, glitazone or glitazar or medications with a lipid-lowering agent in the past 2 weeks, (2) patients who had a history of cardiac abnormalities (myocardial infarction, coronary artery bypass graft, percutaneous transluminal coronary angioplasty, unstable angina or heart failure, hypertension (>150/100 mmHg), (3) patients with thyroid dysfunction, (4) patients having hepatic dysfunction (aspartate aminotransferase/alanine aminotransferase ≥2.5 times of upper normal limit [UNL] or bilirubin >2 times of UNL), 5) patients with renal dysfunction (serum creatinine >1.2 mg/dL), 6) patients with comorbid serious illness such as tuberculosis, HIV and malignancy, (7) patients on alcohol or drug abuse, (8) patients allergic to the study medications and (9) pregnant female patients and nursing mothers. All subjects provided written informed consent before randomisation of the study medication.
A total of 40 patients were recruited. The patients after screening were allocated according to a random computer-generated number into two groups of 20 patients in each group. One group was prescribed with Tablet Saroglitazar 4 mg with Tablet Atrovastatin 10 mg whereas in other group patients were prescribed Tablet Fenofibrate 200 mg with Tablet Atorvastatin 10 mg [Figure 1].
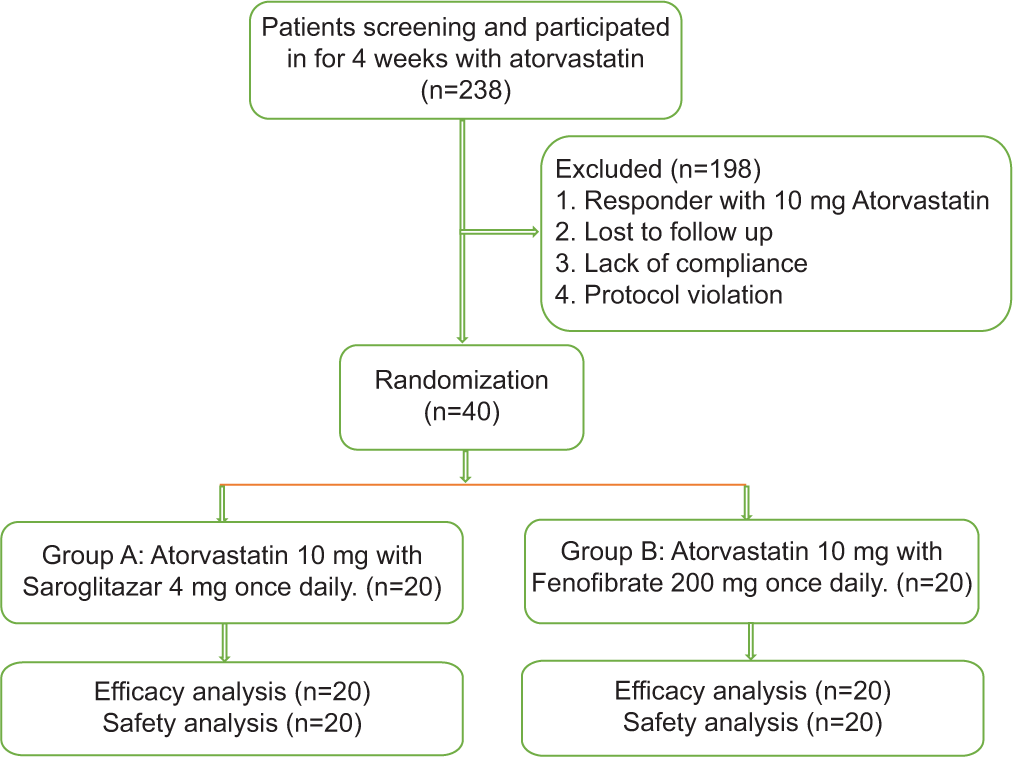
- Flowchart.
Study procedure
Eligibility to participate in the study was assessed by the investigator/physician. Patients went through physical examinations and baseline laboratory examinations. Liver function test (LFT), kidney function test (KFT) and thyroid-stimulating hormone (TSH) examination were undertaken at baseline and the patients with deranged LFT, KFT and TSH were excluded from the study. A pre-designed proforma was filled for each subject which would include the relevant history and investigations. The study was be conducted over a period of 3 months. Patients were investigated for lipid profile, fasting blood glucose (FBG) and HbA1c on day 0 and after completion of 12 weeks. They were asked to report and note down any adverse event during the duration of therapy. They were free to keep in touch by telephone and were contacted every month for their well-being and adherence to prescribed treatment and instructions.
On follow-up visits, on weeks 4, 8 and 12, patients were investigated for lipid profile, FBG and HbA1c. Patients were also asked for adverse effects and went through general examination during follow-up visits.
Primary outcome was absolute change in serum TG level at baseline and at the end of treatment period. Secondary outcome was (a) changes from baseline lipids HDL-C, LDL-C, total cholesterol (TC), VLDL-C, FBG and HbA1c at the end of treatment period (b) safety assessment and glycaemic changes during the duration of study.
Statistical analysis
The collected data were transformed into variables, coded and entered in Microsoft Excel. Data were analysed and statistically evaluated using SPSS-PC-19 version.
Quantitative data were expressed in mean±standard deviation or median with inter quartile range and differences between two comparable groups were tested by Student’s t-test (unpaired) or Mann–Whitney ‘U’ test while Qualitative data were expressed in percentage and statistical differences between the proportions were tested by Chi-square test or Fisher’s exact test. ‘P’ < 0.05 was considered statistically significant. Since it was a short duration pilot study, sample size estimation was not undertaken and a sample size of 40 was finalised.
RESULTS
A total of 238 diabetic patients were screened and investigated for lipid profile, patients with deranged lipid profile and hyperglycaemia were randomly divided into two groups [Figure 1]. Baseline demographic, clinical and laboratory characteristics of the study population after the run-in period were comparable across the treatment groups [Table 1].
| Demographics | Saroglitazar 4 mg with atorvastatin 10 mg. Mean (%) n=20 | Fenofibrate 200 mg with atorvastatin 10 mg. Mean (%) n=20 |
|---|---|---|
| Female (%) | 17 (85) | 15 (75) |
| Male (%) | 3 (15) | 5 (25) |
| Age (year) | 46.55±7.74 | 48.25±7.12 |
| BMI | 26.1±3.62 | 26.4±3.12 |
| Laboratory data* | ||
| TG (mg/dL) | 284.75±50.05 | 283.60±46.11 |
| LDL (mg/dL) | 103.05±33.42 | 100.90±33.48 |
| HDL (mg/dL) | 34.15±6.23 | 34.10±5.78 |
| VLDL (mg/dL) | 51.90±6.77 | 52.45±7.31 |
| Cholesterol | 207.85±23.13 | 213.50±17.67 |
| FBS | 155.90±22.32 | 140.65±27.14 |
| HbA1c | 8.01±0.57 | 7.93±0.57 |
Baseline characteristics
Patients with similar demographics, disease status and medications were balanced in both the treatment arms. All patients in both the groups were on Metformin. Other anti-diabetic medications were sulphonylureas, DPP-4 inhibitors and Voglibose. The subjects were equally distributed with regard to baseline characteristics [Table 1].
Efficacy analysis
The primary end point of the study was the change in TG levels from baseline to the end of week 12. At baseline, mean TG level in Group A (Atorvastatin 10 mg with Saroglitazar 4 mg) was found to be 284.75 ± 50.05 which decreased to 158.95 ± 24.96 at week 12 and in Group B (Atorvastatin 10 mg with Fenofibrate 200 mg), TG level at baseline was found to be 283.60 ± 46.11 which decreased to 174.95 ± 29.63 at week 12. Mean change from baseline to the end of treatment that is, week 12 in Group A was 125.80 ± 45.70 and in Group B, it was found to be 108.65 ± 28.25 [Figure 2]. The difference in mean change of TG levels in both the groups was statistically not found to be significant (P = 0.16).
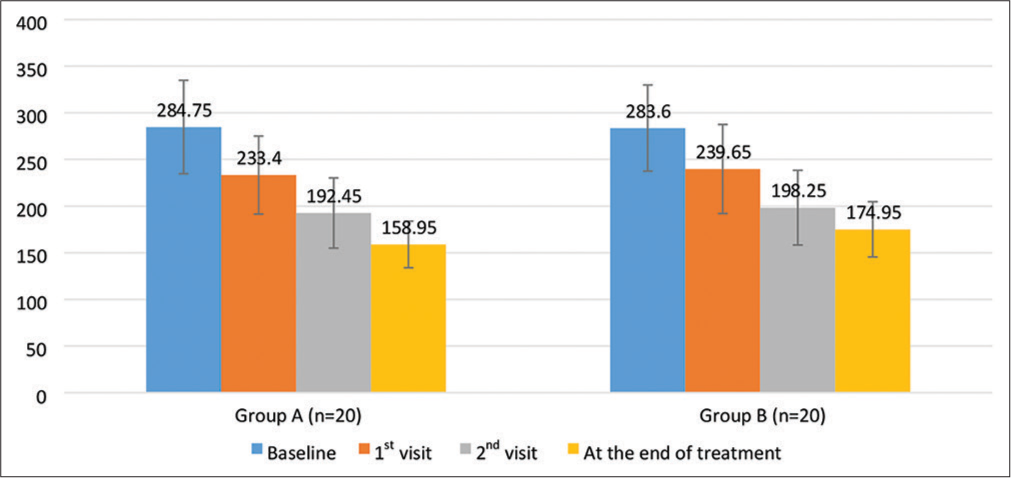
- Changes in triglyceride levels during course of study.
Secondary efficacy end point
Mean TC level in Group A was found to be 207.85 ± 23.13 which decreased to 150.10 ± 16.22 at the end of treatment and in Group B at baseline cholesterol level was found to be 213.50 ± 17.67 which decreased to 145.55 ± 14.40 at week 12. Mean change in levels of TC from baseline to the end of treatment that is, week 12 in Group A was 57.75 ± 21.18 and in Group B was found to be 67.95 ± 14.25. The difference in mean change of TC levels in both the groups statistically was not found to be significant (P = 0.08). Mean change in levels of LDL from baseline at the end of treatment that is, week 12 in Group A was 35.90 ± 13.76 and in Group B found to be 42.50 ± 16.87 at week 12. The difference in mean change of LDL levels in both the groups statistically was not found to be significant (P = 0.18). Mean change in levels of HDL from baseline to the end of treatment that is, week 12 in Group A was 11.25 ± 6.68 and was found to be 9.25 ± 4.24 in Group B. The difference in mean change of HDL levels in both the groups statistically was not found to be significant (P = 0.26). Mean change in levels of VLDL from baseline to at the end of treatment that is, week 12 in Group A was found to be 13.45 ± 6.76 and 14.60 ± 6.89 in Group B. The difference in mean change of VLDL levels in both the groups statistically was not found to be significant (P = 0.59) [Table 2].
| Laboratory parameters | Mean change from Baseline at the end of treatment |
P-value* | |
|---|---|---|---|
| Saroglitazar 4 mg and Atorvastatin 10 mg (n=20) | Fenofibrate 200 mg and Atorvastatin 10 mg (n=20) | ||
| Total Cholesterol (mg/dL) | 57.75±21.18 | 67.95±14.25 | 0.08 |
| TG level (mg/dL) | 125.80±45.70 | 108.65±28.25 | 0.16 |
| LDL level (mg/dL) | 35.90±13.76 | 42.50±16.87 | 0.18 |
| HDL level (mg/dL) | 11.25±6.68 | 9.25±4.24 | 0.26 |
| VLDL level (mg/dL) | 13.45±6.76 | 14.60±6.89 | 0.59 |
| HbA1C level (%) | 1.20±0.59 | 0.26±0.45 | <0.01 |
| FBS level (mg/dL) | 45.15±21.02 | 26.95±23.18 | 0.01 |
TG: Triglyceride, LDL: Low-density lipoprotein, HDL: High-density lipoprotein, VLDL: Very low-density lipoprotein, FBS: Fasting blood sugar, HbA1c: Glycosylated haemoglobin. *Student’s t-test
Mean HbA1c levels in Group A were found to be 8.01 ± 0.57 and decreased to 6.81 ± 0.80 at week 12. In Group B, at baseline, HbA1c level was found to be 7.93 ± 0.57 and decreased to 7.67 ± 0.47 at week 12. Mean change in levels of HbA1c from baseline to the end of treatment that is, week 12 in Group A was 1.20 ± 0.59 and 0.26 ± 0.45 in Group B, respectively. The difference in mean change of HbA1c levels in both the groups statistically was found to be significant (P< 0.01). Mean change in levels of FBS from baseline to the end of treatment that is, week 12 in Group A was found to be 45.15 ± 21.02 and 26.95 ± 23.18 in Group B, respectively. The difference in mean change of FBS levels in both the groups statistically was found to be significant (P = 0.01) [Table 2].
Safety analysis
In all, three of 20 patients reported adverse events in Group A (Atorvastatin 10 mg with Saroglitazar 4 mg), while six of 20 patients reported adverse events in Group B (Atorvastatin 10 mg with Fenofibrate 200 mg). The reported adverse events were mild in nature such as bodyache, weakness and nausea [Table 3]. There was no serious drug event reported in both the groups. Treatment protocol was not altered as adverse events were self-limiting.
| Side effects | Group A (n=20) | Group B (n=20) | P-value* | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Bodyache | 1 | 5.0 | 2 | 10.0 | 0.99 |
| Nausea | 0 | 0.0 | 1 | 5.0 | 0.99 |
| Gastritis | 1 | 5.0 | 1 | 5.0 | - |
| Weakness | 1 | 5.0 | 2 | 10.0 | 0.99 |
DISCUSSION
In our study, we observed potential role of Saroglitazar in treating dyslipidaemia and hyperglycaemia in patients with DD. DM is associated with a considerably increased risk of premature atherosclerotic cardiovascular risk. Dyslipidaemia is a common feature of diabetes. There is an association between atherosclerotic cardiovascular disease and serum lipid levels. The lipid profile in patients of DD consists of mild to marked elevation of VLDL and VLDL remnants concentration and low level HDL cholesterol with elevated TGs. LDL-C levels in diabetic patients are not usually significantly increased compared with those in non-diabetic patients.[10]
In this 12 week study, we observed that Saroglitazar 4 mg in combination with low dose of Atorvastatin 10 mg has better outcome with regard to glycaemic control in comparison to Fenofibrate 200 mg with low dose of Atorvastatin 10 mg. We also observed that dyslipidaemia was effectively controlled in both the groups as the difference in decrease in lipid levels was not found to be statistically significant. Similar findings were observed in a prospective and randomised study (PRESS V), which was the first confirmatory clinical study of Saroglitazar. In PRESS V trial, Saroglitazar produced dose-related decrease in TG levels at 2 mg and 4 mg.[11] In efficacy analysis, Saroglitazar 2 mg and 4 mg significantly reduced plasma TG from baseline by 26.4% and 45%, respectively. Treatment with Saroglitazar 4 mg in PRESS V trial demonstrated marked decrease in low-density lipoprotein (5%), very low-density lipoprotein (45.5%) and TC (7.7%) as compared to pioglitazone at week 24.[11]
In our study, we observed that in the arm administered with Saroglitazar 4 mg, difference in decrease in TG levels statistically was not significant between Saroglitazar and Fenofibrate group. This finding in our study was comparable with already published study.[11] We also observed that Saroglitazar decreased TC by 28% in contrast to Fenofibrate where the decrease in TC levels was found to be 32%. Hence, in our study, Saroglitazar was found to be as effective as Fenofibrate in reducing TC levels in patients of DD. These findings are in contrast with PRESS-V study where the decrease in TC levels was found to be 7.7% only. The possible reason for this difference could be absence of statin therapy from PRESS-V trial.
In a multi-centre, randomised and double-blind study (PRESS VI trial), safety and efficacy of Saroglitazar were compared with placebo in patients of T2DM with dyslipidaemia not controlled with atorvastatin therapy.[12] Significant decrease in TG and TC levels with Saroglitazar therapy was observed. Similar findings with regard to decrease in levels of TG and TC (44% and 28%, respectively) were observed in our study.
We observed that mean decrease in LDL, VLDL and HDL levels was 35%, and 26% and mean increase in HDL levels were 25%, respectively, in our study. These findings are comparable with previously published PRESS-V trials where in mean decrease in LDL, and VLDL was found to be 5% and 45%, respectively. The mean increase in HDL levels in PRESS-V trial was found to be 4%, this is in contrast to our study where increase in HDL levels was 25%. Possible reason for this difference in increased HDL levels in our study is concomitant administration of Atorvastatin. In another study, that is, PRESS-VI trial, mean decrease in LDL, VLDL and HDL levels was 33%, and 48% and mean increase in HDL levels were 33%, respectively. We observed similar findings in our study, as statins were given in both the studies along with Saroglitazar.
In our study, we observed that with Saroglitazar, mean decrease in fasting blood sugar was found to be 27% as compared to the baseline value. Mean percent decrease in level of HbA1c was found to be 14% in our study. Our findings of effect on glycaemic control with Saroglitazar are comparable with PRESS-VI study.[12] In an observational study of effects of Saroglitazar on glycaemic and lipid parameters, it has been reported that mean percent decrease in HbA1c was 6% with Saroglitazar and fall in FBG level was by 23%.[13] These findings are comparable to our study with regard to glycaemic control.
In PRESS-V trial, Saroglitazar 4 mg was found to be comparable to pioglitazone 45 mg in controlling FBG and HbA1c. In addition, Saroglitazar seems to be safe and well tolerated over a course of 24 weeks.[11] No serious adverse effect was reported in our study and the previously published literature with Saroglitazar. Although sample size of our study was small to make any definitive conclusions. No adjustment in doses of atorvastatin and other antidiabetic medications was done over study period.
CONCLUSION
Saroglitazar, a dual PPAR-α/γ agonist, is a potential therapeutic option for the management of DD. It has dual benefit of significant improvement in glycaemic parameters (HbA1C and FBG) as add on therapy to other antidiabetic medication and improvement in dyslipidaemia. The results of our study indicate that Saroglitazar is a promising drug in the management of DD. Considering antidyslipidaemic and antiglycaemic effects, saroglitazar has the potential to address the challenges of reduction of macrovascular and microvascular events in larger outcome studies. However, further prospective research is required to establish this fact. Robust post-marketing surveillance and cohort studies are required to establish its safety and efficacy.
Limitations
Small sample size and short duration of study could have confounding effects on the outcome of the study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Prevalence of dyslipidemia in adult Indian diabetic patients: A cross sectional study (SOLID) Indian J Endocrinol Metab. 2014;18:642-7.
- [CrossRef] [PubMed] [Google Scholar]
- Dyslipidemia in Type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab. 2009;5:150-9.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanisms of diabetic dyslipidemia: Relevance for atherogenesis. Curr Vasc Pharmacol. 2012;10:684-6.
- [CrossRef] [PubMed] [Google Scholar]
- ESC/EAS Guidelines for the management of dyslipidaemias: The task force for the management of dyslipidaemias of the European society of cardiology (ESC) and the European atherosclerosis society (EAS) Eur Heart J. 2011;32:1769-818.
- [Google Scholar]
- Safety of statins: Effects on muscle and the liver. Cleve Clin J Med. 2005;72:990-3, 996-1001
- [CrossRef] [PubMed] [Google Scholar]
- Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: A meta-analysis. JAMA. 2011;305:2556-64.
- [CrossRef] [PubMed] [Google Scholar]
- Fibrates: What have we learned in the past 40 years? Pharmacotherapy. 2007;27:412-24.
- [CrossRef] [PubMed] [Google Scholar]
- Essential of Medical pharmacology (8th ed). New Delhi: Jaypee Brothers; 2018. p. :688.
- [Google Scholar]
- Screening for dyslipidemia. Practice parameter. Am J Clin Pathol. 1995;103:380-5.
- [CrossRef] [PubMed] [Google Scholar]
- A multicenter, prospective, randomized, double-blind study to evaluate the safety and efficacy of Saroglitazar 2 and 4 mg compared to pioglitazone 45 mg in diabetic dyslipidemia (PRESS V) J Diabetes Sci Technol. 2014;8:132-41.
- [CrossRef] [PubMed] [Google Scholar]
- A multicenter, prospective, randomized, double-blind study to evaluate the safety and efficacy of Saroglitazar 2 and 4 mg compared with placebo in Type 2 diabetes mellitus patients having hypertriglyceridemia not controlled with atorvastatin therapy (PRESS VI) Diabetes Technol Ther. 2014;16:63-71.
- [CrossRef] [PubMed] [Google Scholar]
- Observational study of effects of Saroglitazar on glycaemic and lipid parameters on Indian patients with Type 2 diabetes. Sci Rep. 2015;5:7706.
- [CrossRef] [PubMed] [Google Scholar]






