Translate this page into:
Nerve conduction study in young children suffering from cerebral palsy
*Corresponding author: Priyanka Bhagat, Department of Physiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India. pbphysio@bhu.ac.in
-
Received: ,
Accepted: ,
How to cite this article: Basak I, Tiwari AK, Prasad R, Pandey R, Singh TB, Mandal MB, et al. Nerve conduction study in young children suffering from cerebral palsy. Indian J Physiol Pharmacol 2023;67:118-24.
Abstract
Objectives:
Cerebral palsy (CP) is a motor impairment syndrome leading to disorders of movement and posture. Screening of electrophysiological parameters Hoffman reflex (H-reflex and nerve conduction velocities) becomes a necessary for the early detection and management of the disease. The study aimed to assess the various electrophysiological parameters of nerve conduction velocity in CP and age-matched normal children.
Matrials and Methods:
The present cross-sectional study was performed between children suffering from CP and healthy control. A total of 27 children of 12–24 months of age of either sex were examined. Among them, six children were normal (with age match), seven were diagnosed with spastic CP and remaining 14 children were diagnosed with hypotonic CP The electrophysiological parameters were recorded in the right lower limb (posterior tibial nerve-soleus muscle) of all children.
Results:
In electrophysiological parameters, H-reflex latency in secs values was significantly decreased in all CP children. The maximum amplitudes of reflexly excitable motor neurons (Hmax) (mV) and Hmax/maximum amplitude of motor response ratio in the gastrocnemius-soleus muscle were significantly increased in spastic CPas compared to control. H-reflex conduction velocity (HRCV) was significantly higher than motor nerve conduction velocity (MNCV) in hypotonic CP children.
Conclusion:
The electrophysiological parameters were altered in spastic CP children. The electrophysiological parameters in hypotonic CP were within range, indicating they did not suppress the neuronal motor pool. However, HRCV was significantly more than MNCV in hypotonic CP, suggesting some myelination process defect/white matter injury in motor neurons. We concluded that the electrophysiological parameters of the nerve conduction study are a reliable test for the assessment of tone of muscles in children. Thus, it may help in the early initiation of the treatment and therapies in CP children.
Keywords
Hoffman reflex
Motor nerve conduction velocity
Hoffman reflex conduction velocity
Cerebral palsy
INTRODUCTION
Cerebral palsy (CP) is the common cause of motor disability in childhood. It occurs in about 2.1/1,000 live births.[1] William Little first described CP in the 1840s as ‘a group of non-progressive but often altering motor impairment syndromes secondary to lesions or anomalies of the brain arising in the early stages of its development’.[2] It is characterised by abnormal muscle tone, posture and movement, thereby limiting the motor activity of the affected children.[1] The clinical presentation may change as the central nervous system’s growth, development and maturation progress.[1] The motor disorders of CP are often accompanied by disturbances of sensation, perception and secondary musculoskeletal problems.[3]
Early detection of muscle tone in CP is important as the management of diseases, physiotherapy and occupational therapy solely depend on the degree of muscle tone. Identifying the degree of spasticity/muscle tone in younger CP children is still a challenging problem in the early stages of diseases. Examining the deep tendon reflexes is subjective rather than quantitated assessment.
Most of the previous reports on CP are concerned with aetiology and treatment.[1,4-6] Few studies deal with quantitative evaluation of reflex and voluntary activity in children with spasticity CP and state that increased/abnormal reflexes are related to functional impairment in spastic CP.[7] Some electrophysiological studies were performed on the newborn with birth asphyxia but not in cases of CP and reported suppressed electrophysiological parameters.[8,9] A study in older children with spastic CP reported positive correlation between spasticity assessed by the modified Ashworth scale and Hoffman reflex (H-reflex).[10] The excitability properties of nerves in adult CP patients were reported to be abnormal. The altered motoneurons excitability anticipates altered axons conduction properties.[5] The effect of altered excitability of neurons on their nerve conduction properties remains to be explored in patients suffering from CP.
It has been known that the study of peripheral nerve pathophysiology is limited to nerve conduction. The effect of excitability of neurons on conduction is also important to understand the diseases. The electrophysiological examination, that is, H-reflex, may help assess the excitability status of neurons in this situation. H-reflex is the electrical analogue of the monosynaptic stretch reflex. It helps to assess the velocity-dependent increase in muscle stretch reflexes and spinal cord circuits quantitatively.[11] The electrophysiological parameter, for example, maximum amplitudes of reflexly excitable motor neurons (Hmax), maximum amplitude of motor response (Mmax) and Hmax/Mmax ratio in % represents the proportion of reflexly excitable motor neurons.[11] H-reflex latency (HRL) measures the total traversing time of the nerve impulse along the monosynaptic spinal reflex arc, whereas motor response latency (MRL) measures the time taken by the muscle to initiate a compound muscle action potential (CMAP) after direct stimulation of distal motor segment of the nerve. The H-reflex conduction velocity (HRCV) assesses the conduction in the proximal segment of the reflex arc, and motor nerve conduction velocity (MNCV) assesses the conduction in the distal part of the motor neuron.[12] Therefore, above parameters help to assess the excitability and conductivity of the nerves.
This study was conducted to assess the alteration of electrophysiological parameters of nerve conduction velocity study in CP and age-matched normal children.
MATERIALS AND METHODS
The present cross-sectional study was performed on children suffering from CP. The period of study was from November 2018 to October 2020. The study protocol was duly approved by the Ethical Committee of the Institute of Medical Sciences, Banaras Hindu University, Varanasi, India, with IRB number Dean/2018/EC/852, ECR/Bhu/Inst/UP/2017/RR-20.
Study design
The study comprised 27 children from 12 to 24 months of age of either sex who attended the Out Patient Department (OPD) of Paediatrics Medicine of Sir Sunderlal Hospital, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India. Out of them, six children were normal, seven were diagnosed with spastic CP and the remaining 14 were diagnosed with hypotonic CP. The various electrophysiological parameters were recorded in normal, spastic and hypotonic CP children and compared with normal children.
Selection criteria of subjects
Inclusion criteria
Children with CP who were born full-term appropriate for gestational age with birth weight 10th percentile of Indian local standard for their gestation age were selected for study.[13] Further, healthy children of the same age (of either sex) were selected to serve as the control group. Only hemodynamically stable CP children were recruited for the study.
Exclusion criteria
Children born from diabetic mothers and those who suffered from septicaemia, meningitis, hypoglycaemia or other congenital malformations or chromosomal anomalies were excluded. Children who had recent seizures, received systemic anti-spasticity/anti-convulsion medications or who had received phenol and/or botulinum toxin type A injections, or had past surgical interventions were excluded from the study.
Recording of electrophysiological parameters
The electrophysiological examinations were conducted at the Neurophysiology Research Unit of the Department of Physiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India. Before investigations, bilingual (Hindi/English) written consent was obtained from the parents in a standard consent form approved by the Ethical Committee. This was in compliance with the Declaration of Helsinki (1975), as revised in 2000.
The instruments used in the study: Biopac Student Laboratory Advanced System (Biopac System INC. USA) with filter setting between 5 Hz and 5000 Hz, and GRASS Stimulator model S88 (GRASS Technologies, USA).
Before recording the electrophysiological parameters, anthropometric parameters like height were recorded in each subject. Further, care was taken that the children should be in a hemodynamically stable state.
For electrophysiological examination, children were comfortably placed in a prone position with their legs extended. After proper skin cleaning with soap water and spirit, conducting jelly was applied, and surface electrodes made of silver chloride (Ag/Ag-Cl) were firmly placed over the skin with the help of adhesive tape.
Recording of H-reflex and its parameters: The detailed procedure for recording H-reflex parameters has been described earlier.[8,9,14] Briefly, the active recording electrode was placed on the belly of the soleus muscle in the midline, at the junction of the upper 2/3rd and lower 1/3rd of the calf region. The reference electrode was placed just posterior to the medial malleolus. The ground electrode was placed over the soleus muscle between the stimulating and the active recording electrodes. The stimulating electrode was placed on the skin above the posterior tibial nerve in popliteal fossa after applying conducting jelly. The cathode was placed mid in the popliteal fossa, and the anode was placed on lateral side of the knee. The above recording site was selected to achieve the maximum reflex excitability of motor neurons with the least variation on the serial recording. Single square wave stimulus with 0.1–0.2 ms duration was delivered percutaneously on the posterior tibial nerve at the midpopliteal region. The elicited CMAP was displayed on a monitor of data acquisition system. The placement of the electrodes on the limb was kept constant in all the subjects as electrophysiological parameter values may alter with electrode position. The submaximal strength of stimulus was used for eliciting H-reflex since it activates the Ia sensory fibres.[15] The supramaximal strength of the stimulus was used for recording the maximum motor response. It stimulates α motor neurons.[9,15]
H-reflex conduction velocities were calculated as per the formula given by Vecchierini-Blineau and Guiheneuc.[12]
HRCV (m/s) = 0.8 × height (in mm)/(HRL – MRL [in ms])−1
In this formula, the distance travelled by nerve impulse in the soleus H-reflex pathway has been calculated as 80% of the height of children. The conduction time in the proximal segment of the reflex arc is calculated with the start point from the popliteal fossa (Ia afferent) and the endpoint again at popliteal fossa (α motor neuron), that is, HRL−MRL. The central synaptic delay time is taken as 1 ms, which was deducted to calculate HRCV.[12]
Recording of MNCV: for this recording, the active electrode is placed over the belly of the abductor digiti minimi muscle on the lateral aspect of the sole. The MNCV was determined by stimulating the right posterior tibial nerve at two different points along its course: the popliteal fossa and behind the medial malleolus.[14]
It was calculated as per the formula; MNCV (m/s) = distance/ time.
In this formula, distance refers to the distance between two stimulating points, that is, popliteal fossa to medial malleolus and time is a difference in latencies.
Statistical analysis
Arithmetic mean and standard deviation was calculated for quantitative variables. As the data were not normally distributed and the number of cases in each group was small, the differences in various corresponding parameters between groups were evaluated for statistical significance using the non-parametric Mann–Whitney U-test. P < 0.05 was considered significant. SPSS 16.0, Sigma Plot 10 and MS Excel were used for statistical and graphical analyses.
RESULTS
In the present study, [Figures 1 and 2] depicts the labelled original recording of H-reflex parameters and MNCV, respectively.
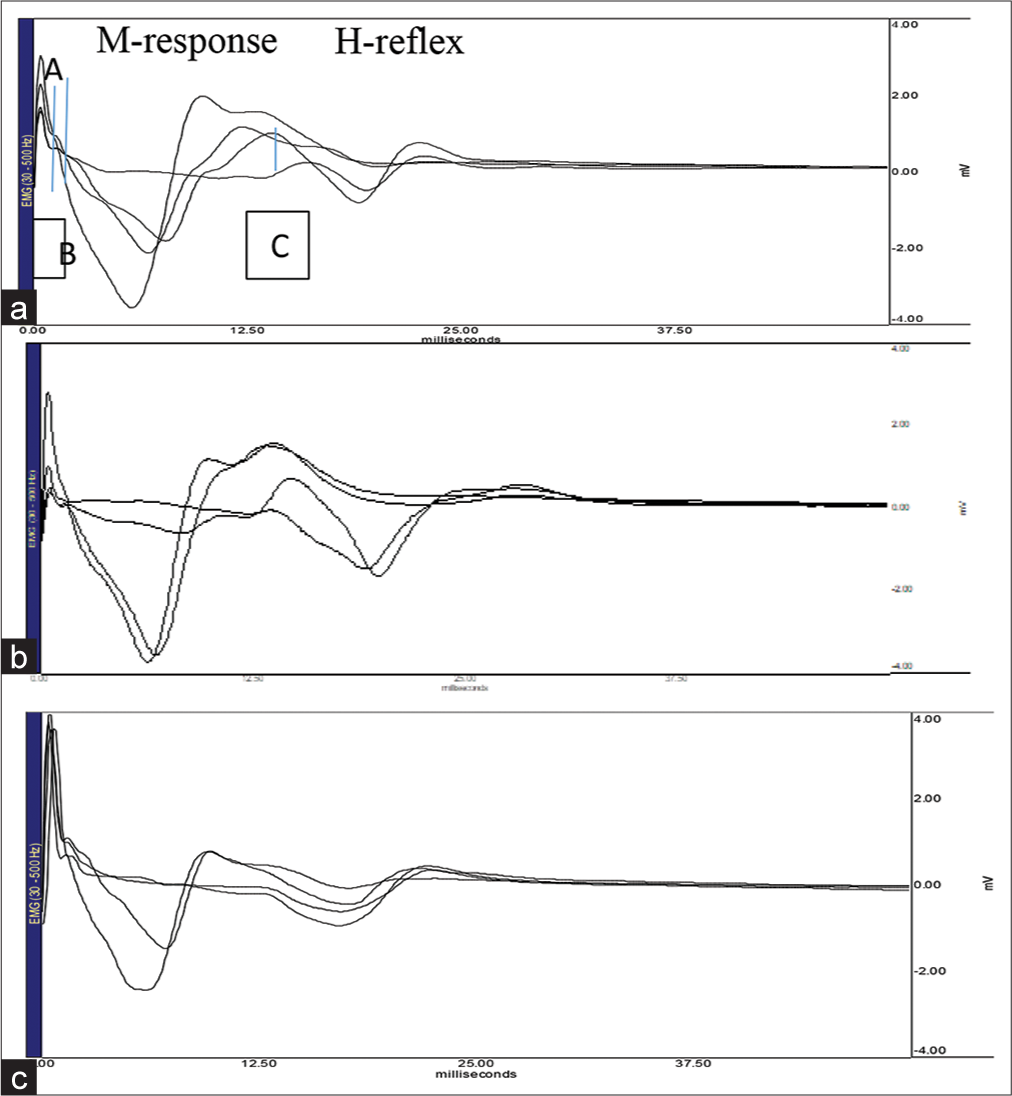
- Above recordings shows Hoffman reflex (H-reflex) and M response (Motor response) in right lower limb (posterior tibial nerve-soleus muscle) of normal and cerebral palsy (CP) children. Horizontal axis denotes time (ms) and vertical axis represents amplitude (mV). Top panel (a) – Normal children; tracing shows: M response and H-reflex waves; AB: M response latency, AC: H-reflex latency. Middle panel (b) – spastic CP children. Bottom panel (c) – hypotonic CP children.
- Sample recording of H-reflex parameters in normal and CP children

- Above recording shows MNCV by stimulating the posterior tibial nerve at two different points along its course, the popliteal fossa and behind the medial malleolus and position of active electrode over the belly of the abductor digiti minimi muscle on the medial aspect of the foot. Top panel (a) – normal children, middle panel (b) – spastic cerebral palsy (CP) children, bottom panel (c) – hypotonic CP children, MNCV: Motor nerve conduction velocity.
- Sample recording of MNCV in normal and CP children
H-reflex was elicited in all the normal children. The values of H reflex parameters and MNCV in normal children were within the normal limit of their respective age group [Table 1].
| Electrophysiological parameter |
Control (n=6) | Spastic CP (n=7) |
|---|---|---|
| HRL (s) in soleus muscle | 0.017±0.004 | 0.013±0.004* |
| Hmax (mV) in soleus muscle |
1.161±0.55 | 3.96±2.43* |
| MRL (s) in soleus muscle | 0.003±0.001 | 0.006±0.004 |
| Mmax (mV) in soleus muscle |
1.76±1.33 | 1.45±0.85 |
| H/M ratio | 65.90±32.70 | 273.63±132.29* |
| HRCV (m/s) | 66.38±15.04 | 63.75±11.34 |
| MNCV (m/s) | 65.83±15.01 | 61.714±10.43 |
Above table shows mean±standard deviation values of electrophysiological parameters of spastic CP and normal children. CP: Cerebral palsy, HRL: H-reflex latency, Hmax: H-reflex amplitude, MRL: M response latency, Mmax: M response amplitude, HRCV: H-reflex conduction velocity, MNCV: Motor nerve conduction velocity. “n” denote the number of subjects in each group. *indicates significant difference between the values (P<0.05; non parametric Mann– Whitney U-test). Values of other parameters (MRL, Mmax, HRCV and MNCV) were not significantly different from control values (P>0.05; non-parametric Mann–Whitney U-test)
In spastic CP children, the values of HRL (0.013 ± 0.004 s, P < 0.05) were significantly lower in comparison to normal children, whereas the values of Hmax (3.96 ± 2.43 mV, P < 0.05) and Hmax/Mmax ratio (282.63 ± 132.29%, P < 0.05) were significantly higher as compared to normal children [Table 1]. Other electrophysiological parameters, HRCV (63.75 ± 11.34 m/s) and MNCV (61.714 ± 10.43 m/s), were similar to the control group. Correspondingly, the values of HRCV were similar to MNCV [Table 1].
In hypotonic CP children, the values of HRL (0.007 ± 0.004 s, P < 0.05) were significantly lower as compared to normal children [Table 2]. Other electrophysiological parameters such as Hmax (1.083 ± 0.38 mV), Mmax (3.01 ± 1.92 mV), MRL (0.003 ± 0.001 s) and Hmax/Mmax ratio (46.09 ± 24.08%) were similar to normal children. HRCV (63.78 ± 14.13 m/s) and MNCV (56.71 ± 13.65 m/s) were also similar to normal children. However, HRCV was significantly (P < 0.05) higher than MNCV in this group [Table 2].
| Electrophysiological parameter | Control (n=6) | Hypotonic CP (n=14) |
|---|---|---|
| HRL (s) in soleus muscle | 0.017±0.004 | 0.007±0.004* |
| Hmax (mV) in soleus muscle | 1.161±0.55 | 1.083±0.38 |
| MRL (s) in soleus muscle | 0.003±0.001 | 0.003±0.001 |
| Mmax (mV) in soleus muscle | 1.76±1.33 | 3.01±1.92 |
| H/M ratio | 59.45±32.70 | 46.09±24.08 |
| HRCV (m/s) | 66.38±15.04 | 63.78±14.13 |
| MNCV (m/s) | 65.83±15.01 | 56.714±13.65# |
Above table shows mean±standard deviation values of electrophysiological parameters of Hypotonic CP and normal children. CP: Cerebral palsy, HRL: H-reflex latency, Hmax: H-reflex amplitude, MRL: M response latency, Mmax: M response amplitude, HRCV: H-reflex conduction velocity, MNCV: Motor nerve conduction velocity. “n” denote the number of subjects in each group. *indicates significant difference between the values (P<0.05; non parametric Mann– Whitney U-test), #indicates MNCV significantly less compared to HRCV in same group
DISCUSSION
In the present study, H-reflex was elicited in all CP and healthy children [Figure 1]. Hmax and Hmax/Mmax ratios were significantly higher in spastic CP children compared to the control group, whereas these parameters were not significantly different from normal children in hypotonic CP [Tables 1 and 2]. HRL was significantly shorter in all CP children. Proximal conduction velocity, that is, HRCV was significantly higher than distal motor conduction velocity, that is, MNCV in hypotonic CP children [Table 2].
Perinatal hypoxia and other prenatal causes were considered the major cause of CP.[16] Injury or deformity at an earlier brain development stage, mainly of the basal ganglia and cerebellum, has been reported as an important cause of hypotonic CP.[4,6] Further, generalised muscular hypotonia observed in hypotonic CP may not result from a primary disorder of muscle and peripheral nerves.[6,15] Reports suggested that pyramidal tract disorders are observed in spastic CP. Furthermore, disrupted descending inputs may affect peripheral nerves and muscle fibres resulting in alteration of functions of concerned organ.[5] In our observations, H-reflex was elicited in both types of CP children. Thus, all the components of H-reflex arc were anatomically intact and functional despite brain injury in patients with CP.
The present study observed a significant increase in Hmax and Hmax/Mmax ratio in spastic CP children [Table 1]. The increase in Hmax and H/M ratio suggested that the spinal motor neuron excitability was higher in the CP patients. Hagglund and Wagner studied the development of spasticity with age using the Ashworth scale. They reported that muscle tone in CP children gradually increased up to 4 years of age and then gradually decreased till 12 years of age. The same tendency is seen in all CP subtypes.[17] In our study, the age group of patients was from 12 months to 24 months. Thus, above study supported our observation. Another study reported that perinatal damage to the corticospinal pathway secondarily leads to the disrupted development of spinal motor centres or malfunction of the spinal interneuron due to altered corticospinal input.[18] These supra spinal lesions of the pyramidal tract occur before the central nervous system maturation is complete.[18] Thus, in CP patients, disrupted descending inputs may lead to secondary changes in the spinal cord, peripheral nerves and muscle fibres, causing increased excitability in the spinal motor neuronal pool and tone of muscles and contributing to increased Hmax and Hmax/Mmax ratio in spastic CP.
In hypotonic CP, H-reflex parameters, that is, Hmax and H/M ratio were within the normal range, indicating no alteration in excitability in the spinal motor neuron pool. The electrophysiological investigation, that is, H-reflex in hypotonic CP children has not been reported in literature. Studies on other hypotonic disorders such as paralytic poliomyelitis and spinal cord injuries have reported reduced H-reflex parameters, which attributed to damage of the motor neurons in the affected side of the spinal cord.[19] Prakash et al. and Kumar et al. studied electrophysiological parameters on birth hypoxia and foetal distress in newborns.[8,9] They reported that motor neuron excitability of peripheral nerve of the spinal cord was affected by hypoxia in the early life of infancy, and there was transiently depressed spinal reflex excitability in the first 72 h after birth and it returned to normalcy.[8,9] Another study on spinal cord injury reported that loss/suppression of the H-reflex is not immediate but a gradual process involving adaptation throughout the neuromuscular system.[20] These reports supported our findings that H-reflex parameters were not much altered in our study. These finds may be due to mild damage to the brain or due to the age of children. Further, hypotonia may not be marked until the child gets older.
In the present study, HRL was significantly shorter; however, HRCV and MNCV were within the normal range according to their respective age in all CP children [Tables 1 and 2]. HRCV was higher than MNCV in all CP children in our study. However, in hypotonic CP, the HRCV was significantly more than MNCV.
HRCV indicates the conduction in the proximal segment of the reflex arc. It involves both sensory and motor segment of a spinal monosynaptic reflex arc and is used to assess the degree of myelination of the nervous system.[12] However, MNCV indicates the conduction in motor nerves and mainly depends on the thickness of the myelin sheath and the remodelling of nodes of Ranvier.[14] Only a few reports were available in the literature investigating the peripheral sensory and motor nerve conduction velocities in CP. The excitability properties of nerves in the CP subjects were reported as abnormal in studies elsewhere.[5] The motoneurons are hyperexcitable in CP. Abnormal internodal properties of axons were reported in CP adults that may be attributed to the altered architecture of axons. Simultaneously, a reduced brain inhibitory inputs would increase motoneuron excitability and conductivity. The above explains that higher conduction in the H-reflex pathway leads to shorter HRL in CP children.
Despite increased motoneuron excitability and shorter HRL, the HRCV and MNCV were within the normal range in our observations. This suggested adequate myelination of nerve in CP children. The prenatal ischemia in animal models shows hypomyelination, and axonal degeneration persisting in many parts of the brain and is not detected in white matter zones below the primary motor cortex or the corticospinal tracts.[21,22] HRCV was higher than MNCV in all CP children; however, in hypotonic CP, the HRCV was significantly more than MNCV. The previous studies in normal children reported that the HRCV was higher than MNCV.[12,23,24] As the myelination progresses from the spinal root toward the periphery, the value of proximal conduction velocity would be higher than the distal segment in the developing nerve trunk.[25,26] The difference between proximal and motor distal conduction velocities diminishes with age.[25] An increased HRCV than MNCV in hypotonic CP indicates an increased growth spurt in the myelination process in the proximal segment of a reflex arc than distal motor nerves and some myelination process defect/white matter injury in motor neurons. It leads to higher HRCV than MNCV in hypotonic CP. Spinal motor neuron pool excitability could not alter the conduction velocity in peripheral nerves of spastic and hypotonic CP children. Changes in the peripheral sensory and MNCV are unclear because CP is caused by neurological difficulty in the higher brain, not by problems in the peripheral nerves.
This is a preliminary study of a small sample size. We did not observe the electrophysiological parameter in the left lower and upper limbs of CP children as study was fully dependent on the OPD follow-up and cooperation of parents. We could not co-relate magnetic resonance imaging and computed tomography scan with electrophysiological findings. H-reflex parameters have their limitations, that is, the value of H-reflex in this capacity decreases as spasticity increases due to the development of minor contracture and has some limitations for direct measurement of central spasticity and hypotonia in children.
CONCLUSION
In a present study, the electrophysiological parameters, that is, H-reflex and nerve conduction, were altered in CP children. The presence of H-reflex in all CP children suggested that the reflex arc was anatomically and functionally intact. Increased Hmax, Hmax/Mmax ratio and decreased HRL in spastic CP patients suggested the increased excitability of motor neurons; however, HRCV and MNCV were not altered. H-reflex parameters were not altered in hypotonic CP children suggesting that motor neuronal pool was not suppressed. However, HRCV was significantly more than MNCV in hypotonic CP suggesting some myelination process defect/white matter injury in motor neurons. The excitability status of the spinal motor neuronal pool has not altered the conduction velocity in a peripheral nerve in CP. The electrophysiological parameters of nerve conduction study have the potential as a quantitative measure of reflex excitability and conductivity of neurons in CP children. It can be used as a test to assess the treatment response of CP children. It also helps the physician to start the treatment modality in the early childhood so that disabilities arising in CP children may be minimised or prevented.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Cerebral palsy-trends in epidemiology and recent development in prenatal mechanisms of disease, treatment, and prevention. Front Pediatr. 2017;5:21.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral palsy epidemiology: Where are we now and where are we going? Dev Med Child Neurol. 1992;34:547-51.
- [CrossRef] [PubMed] [Google Scholar]
- A report: The definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8-14.
- [Google Scholar]
- Spasticity and its contribution to hypertonia in cerebral palsy. Biomed Res Int. 2015;2015:317047.
- [CrossRef] [PubMed] [Google Scholar]
- Excitability properties of motor axons in adults with cerebral palsy. Front Hum Neurosci. 2015;9:329.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral palsy-definition, classification, etiology and early diagnosis. Indian J Pediatr. 2005;72:865-8.
- [CrossRef] [PubMed] [Google Scholar]
- Quantitative evaluation of reflex and voluntary activity in children with spasticity. Arch Phys Med Rehabil. 2003;84:828-37.
- [CrossRef] [PubMed] [Google Scholar]
- Spinal motor neuron excitability in newborns following fetal distress: Sub-clinical depression revealed by soleus H-reflex. Clin Neurophysiol. 2005;116:2342-7.
- [CrossRef] [PubMed] [Google Scholar]
- Birth hypoxia and spinal reflex in newborn babies. Electromyogr Clin Neurophysiol. 2005;45:59-63.
- [Google Scholar]
- Electrophysiologic assessment of spasticity in children using H-reflex. Turk J Pediatr. 2013;55:519-23.
- [Google Scholar]
- The monosynaptic reflex: A tool to investigate motor control in humans. Interest and limits. Neurophysiol Clin. 2000;30:67-80.
- [CrossRef] [PubMed] [Google Scholar]
- Electrophysiological study of the peripheral nervous system in children. Changes in proximal and distal conduction velocities from birth to age 5 years. J Neurol Neurosurg Psychiatry. 1979;42:753-9.
- [CrossRef] [PubMed] [Google Scholar]
- Studies on fetal growth patterns: Intrauterine growth percentiles for singleton live born babies. Indian Pediatr. 1981;18:647-53.
- [Google Scholar]
- H-reflex and motor nerve conduction studies in growth retarded newborn babies. Neurosci Lett. 2008;432:188-92.
- [CrossRef] [PubMed] [Google Scholar]
- Motoneurone pool and the H-reflex. J Neurol Neurosurg Psychiatry. 1968;31:354-61.
- [CrossRef] [PubMed] [Google Scholar]
- Birth asphyxia is associated with increased risk of cerebral palsy: A meta-analysis. Front Neurol. 2020;11:704.
- [CrossRef] [PubMed] [Google Scholar]
- Development of spasticity with age in a total population of children with cerebral palsy. BMC Musculoskelet Disord. 2008;9:150.
- [CrossRef] [PubMed] [Google Scholar]
- Modulation of soleus H-reflexes during gait in children with cerebral palsy. J Neurophysiol. 2007;98:3263-8.
- [CrossRef] [PubMed] [Google Scholar]
- Electrophysiological studies in children with paralytic poliomyelitis. Electromyogr Clin Neurophysiol. 1995;35:73-6.
- [Google Scholar]
- Low frequency depression of H-reflexes in humans with acute and chronic spinal-cord injury. Exp Brain Res. 2000;133:233-41.
- [CrossRef] [PubMed] [Google Scholar]
- Animal models of cerebral palsy: Hypoxic brain injury in the newborn. Iran J Child Neurol. 2015;9:9-16.
- [Google Scholar]
- Prenatal ischemia deteriorates white matter, brain organization, and function: Implications for prematurity and cerebral palsy. Dev Med Child Neurol. 2016;58:7-11.
- [CrossRef] [PubMed] [Google Scholar]
- The use of the H reflex in serial evaluation of nerve conduction velocity. Electroencephalogr Clin Neurophysiol. 1983;55:82-90.
- [CrossRef] [PubMed] [Google Scholar]
- Study of nerve conduction and late responses in normal Chinese infants, children, and adults. J Child Neurol. 1997;12:13-8.
- [CrossRef] [PubMed] [Google Scholar]
- Quantitative studies on the maturation of central and peripheral parts of individual ventral motoneuron axons. I. Myelin sheath and axon calibre. J Anat. 1978;126:509-33.
- [Google Scholar]
- The growth and myelination of central and peripheral segments of ventral motoneurone axons. A quantitative ultrastructural study. Brain Res. 1976;105:193-211.
- [CrossRef] [PubMed] [Google Scholar]






