Translate this page into:
Preventive interventional strategies mitigate age-associated degeneration of dorsal hippocampal neural cells in naturally ageing mice
*Corresponding author: Kiranmai S. Rai, Department of Basic Medical Sciences, Manipal Academy of Higher Education, Udupi, Karnataka, India. hod.physio@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bhat MS, D’Almeida PM, Prabhu P, Sivakumar G, Dhanushkodi A, Rai KS. Preventive interventional strategies mitigate age-associated degeneration of dorsal hippocampal neural cells in naturally ageing mice. Indian J Physiol Pharmacol. 2024;68:50-6. doi: 10.25259/IJPP_490_2023
Abstract
Objectives:
The objective of this study was to investigate the effectiveness and potential protective effects of various intervention strategies, such as choline and docosahexaenoic acid (Ch-DHA) supplementation, Clitoria ternatea root extract (CTR) supplements, human embryonic kidney stem cell conditioned media (HEK-CM) treatment and exposure to enriched environments (EEs), in mitigating neural cell degeneration in dorsal hippocampal subregions of naturally ageing mice brain.
Materials and Methods:
Twelve–fifteen-month-old CF1 male mice were subdivided (n = 6/group) into normal age-matched control, Ch-DHA supplemented, EE exposed, HEK-CM administered and CTR-supplemented groups. Neuro morphological alterations in the dorsal hippocampus were assessed using cresyl violet staining.
Results:
Higher neural cell degeneration was observed in the CA1–CA3 dorsal hippocampal subregions in normal ageing mice brains. Notably, interventions such as HEK-CM administration, Ch-DHA supplementation, exposure to an EE and CTR supplementation significantly reduced degeneration, particularly in the CA3 and CA2 regions.
Conclusion:
Ch-DHA supplementation and HEK-CM treatment are observed to significantly reduce age-dependent degeneration of dorsal hippocampal CA3 and CA2 neurons in naturally ageing mice compared to EE exposure or CTR supplementation.
Keywords
Ageing hippocampus
Choline and docosahexaenoic acid
Clitoria ternatea aqueous root extract
Enriched environment
HEK cell-conditioned media
INTRODUCTION
The ageing process can cause changes in cognitive functions, even if an individual does not suffer from cerebrovascular disease or Alzheimer’s disease (AD), disorders known to affect cognition.[1] One of the cognitive domains that is particularly impacted by ageing is memory.[2] This suggests that the hippocampal formation, a critical brain region involved in cognitive functions such as storing and retrieving information, may be especially susceptible to the ageing process.
A hippocampus is not a uniform structure but consists of distinct subfields, each with its histological characteristics. These subfields include the subiculum, which has subdivisions such as the pre-subiculum, para-subiculum and subiculum proper, as well as the four cornu ammonis sectors known as cornu ammonis 1 (CA1) - CA4 subregions, and the dentate gyrus. These subfields are functionally interconnected through two primary intra-hippocampal pathways. The polysynaptic intra-hippocampal pathway comprises the entorhinal cortex, dentate gyrus, CA3, CA4, CA1 and the subiculum. Moreover, the entorhinal cortex projects directly to the CA1 and subiculum regions.[3] Despite these tight functional connections, there is evidence indicating that these subfields have specific functional roles. For instance, CA1 appears to be involved in temporal pattern association and intermediate-term memory, whereas CA3 is responsible for tasks such as spatial pattern association, detecting novelty and short-term memory.[4]
Age-related decline in the hippocampal volume is likely the result of a complex interplay of differing biochemical and electrophysiological changes, but the underlying causes of this decline remain largely unknown.[5,6] Studies have reported significant neuronal cell loss in the hippocampus and reduction in hippocampal volume with increasing age.[7-9] In rodents, hippocampal neurogenesis declines with age mainly because neural progenitor cells (NPCs) gradually become inactive and quiescent, and those dividing NPCs may not produce surviving nerve cell progeny.[10,11] Many studies reported similar types of neuronal loss in the hippocampal complex in humans, monkeys and rats.[12-16] According to certain stereology studies, ageing causes a 5–35% neuronal loss in the hippocampus complex’s CA1, CA2, CA3, CA4, dentate granule cells, entorhinal cortex and in the subiculum.[12-16]
Precisely delineating the typical age-related alterations in the hippocampus and pinpointing the optimal interventional approach to mitigate these changes is of utmost importance. This is particularly significant as early-stage neurological disorders such as AD and dementia exhibit a correlation with hippocampal volume loss and mild cognitive impairment. The parallel manifestations pose a challenge in differentiating between the normal ageing process and the initial phases of dementia and AD. The growing concern over the absence of effective treatments that can modify age-related cognitive decline has led to a heightened focus on preventative approaches. The approaches in the present study encompass a range of strategies, including dietary interventions, cellular therapies and environmental adjustments, all aimed at attenuating hippocampal neural cell loss during normal ageing. Therefore, in the current study, we sought to compare the effects of supplementing with choline and docosahexaenoic acid (Ch-DHA), Clitoria ternatea aqueous root extract (CTR), administering human embryonic kidney stem cell conditioned media (HEK-CM) and exposing mice to an enriched environment (EE) in mitigating the natural age-related impairment and degeneration of neural cells in dorsal hippocampal subregions of normal ageing mice.
Research across behavioural, anatomical and gene expression domains collectively provides evidence for a functional division of the hippocampus into three distinct compartments: Dorsal, intermediate and ventral. The dorsal hippocampus, equivalent to the posterior hippocampus in primates, is primarily associated with cognitive functions related to learning and memory associated with navigation, exploration and locomotion. Conversely, the ventral portion (analogous to the anterior hippocampus in primates) is implicated in processes related to emotion and stress.[17]
MATERIALS AND METHODS
Animals
Twelve–fifteen-month-old, middle-aged male Carworth Farm 1 (CF-1) mice were obtained after approval from the Institutional Animal Care and Use Committee (No. IAEC/KMC/51/2022), Manipal Academy of Higher Education (MAHE), Manipal. All experiments were carried out in accordance with guidelines provided by the IAEC and CCSEA, Government of India. All CF-1 male mice obtained for the study were housed in the central animal house research facility, MAHE, Manipal. During the experimental period, four–five mice were housed/caged and bedded in standard polypropylene cages containing paddy husk as bedding material that was replaced every 2 days. Standard laboratory conditions, with temperatures ranging between 23 ± 20°C, humidity (50 ± 5%) and 12 h light–dark cycle, were maintained for all the mice. Pellet feed with a choline content of 1 mg/kilogram, procured from VRK Nutritional Solutions (VRK’s ‘Scientist’s Choice’ Laboratory Animal Diets, Pune) and water ad libitum to these mice.
Experimental protocol
In this study, middle-aged mice were randomly divided into six groups: Normal age-matched control (NAC), ChDHA supplemented group, HEK-CM treated group, EE exposed group and CTR supplemented group. The NAC group remained undisturbed in their home cage for 30 days. The Ch-DHA group received daily doses of 45 mg/kg of choline and 300 mg/kg of DHA for 30 days. The HEK-CM group received intravenous injections of 100 μL of HEKCM for 5 days within the 30-day period. The EE group was exposed to environmental enrichment (EE) for 3–6 h/day for 30 days. Finally, the CTR-supplemented group was fed with CTR at a dosage of 100 mg/kg of body weight/day for 30 days. Following the treatment, mice were given a high dose of ketamine to induce deep anaesthesia and transcardial perfusion was done with PBS, followed by a 4% paraformaldehyde solution. Brains were then removed and stored in a paraformaldehyde solution at 4°C for 48 h for sectioning. 30 μm thick dorsal hippocampal sections were made using a cryostat microtome. Cresyl violet staining was used to count neural cells in specific regions of the dorsal hippocampus. Neural cell quantification was performed using a Motic microscope equipped with a camera. To ensure unbiased counting, the slides were coded to prevent any manual bias during the process. Specifically, degenerating neural cells exhibiting characteristics such as pyknosis (condensation of the nucleus), intense staining, shrinkage and loss of nuclei were counted within the CA1, CA2 and CA3 regions of the dorsal hippocampus under a high-power field (×400). An increasing number of these neural cells were used as a measure for age-related neural cell degeneration.
Statistical analysis
The statistical analysis was conducted by representing the data as mean values with standard error of the mean. To compare the effects of different treatments amongst the groups, we employed a one-way analysis of variance followed by Bonferroni’s post hoc test. A significance level of P < 0.05 was used to determine statistical significance. The statistical software program Statistical Package for the Social Sciences (RRID: SCR_002865) was utilised for all data analysis.
RESULTS
Degenerating neural cells in the ageing dorsal hippocampus
In the present study, the density of shrinking/degenerating neural cells with more lacunae was observed in CA1, CA2 and CA3 dorsal hippocampal subregions in normal ageing mice [Figures 1-6]. Whereas, in all treated groups, the density of degenerating neural cells in the CA3 subregion was observed to be highly significantly reduced (P < 0.0001; Figure 1) when compared to the same in age-matched NAC mice. Moreover, the density of shrinking/degenerating neural cells with lesser lacunae in the CA2 sub-region of the dorsal hippocampus was observed to be significantly lesser in mice groups treated with HEK-CM (P < 0.001; Figure2), supplemented Ch-DHA or exposed to EE (P < 0.01; Figure 2) and administered CTR (P < 0.05; Figure 2) when compared to the same in age-matched NAC mice. In addition, the number of pyknotic neural cells/high power field was observed to be slightly less in the CA1 sub-region of the dorsal hippocampus in brains from mice groups exposed to EE and those treated with HEK-CM when compared to the same in age-matched NAC mice [Figure 1].
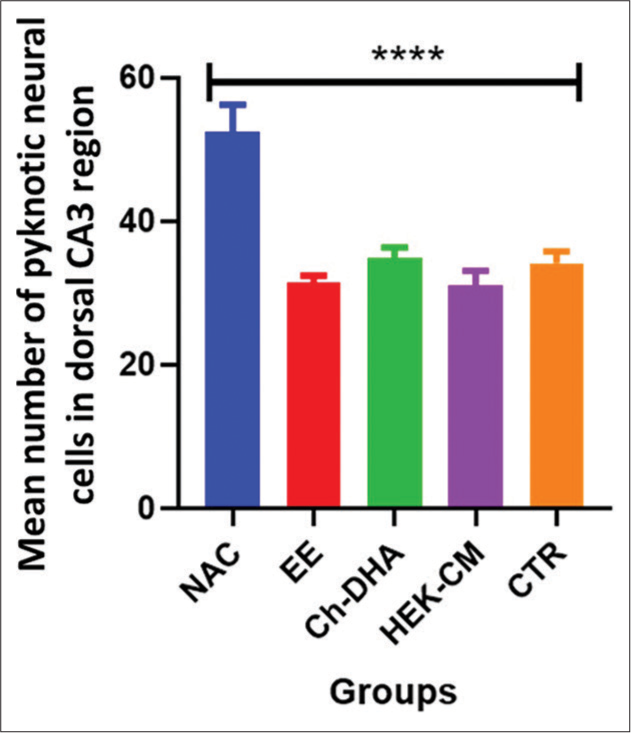
- Degenerating neural cell counts in dorsal hippocampal CA3 region of ageing mice. n = 6/group. Values represent the Mean ± SEM number of pyknotic neural cells in the CA3 region/HPF. A highly significant decrease in the number of pyknotic/degenerating neural cells was found in HEK-CM, Ch-DHA, EE and CTR when compared to the same NAC mice. Comparison between NAC versus HEKCM, Ch-DHA, EE, CTR ****P < 0.0001. CA: Cornu ammonis, SEM: Standard error of the mean, HEK-CM: Human embryonic kidney stem cell conditioned media, ChDHA: Choline and docosahexaenoic acid, EE: Enriched environment, CTR: Clitoria ternatea root extract, NAC: Normal age-matched control.
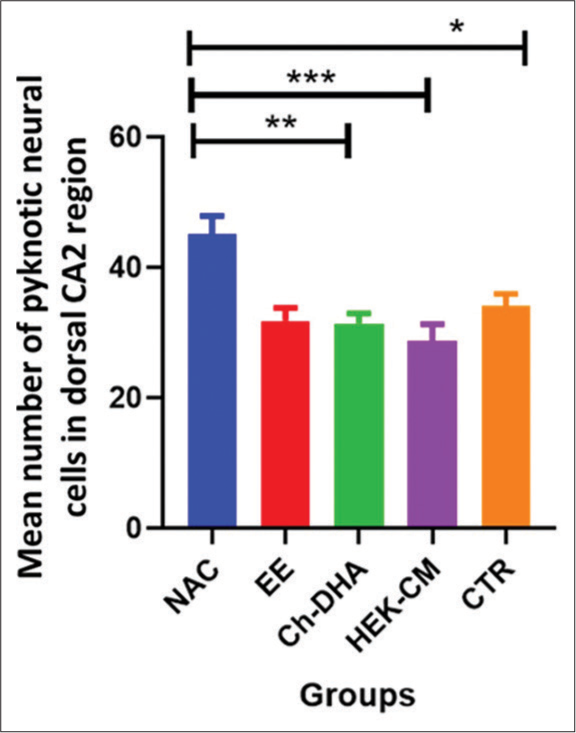
- Degenerating neural cell counts in dorsal hippocampal CA2 region of ageing mice. n = 6/group. Values represent the Mean ± SEM number of pyknotic neural cells in the CA2 region/HPF. A significantly lesser number of pyknotic/degenerating neural cells were found in HEK-CM, Ch-DHA, EE and CTR when compared to the same in NAC mice. Comparison between NAC versus HEK-CM, Ch-DHA, EE, CTR *P < 0.05 **P < 0.01 ***P < 0.01. CA: Cornu ammonis, SEM: Standard error of the mean, HEK-CM: Human embryonic kidney stem cell conditioned media, Ch-DHA: Choline and docosahexaenoic acid, EE: Enriched environment, CTR: Clitoria ternatea root extract, NAC: Normal age-matched control.
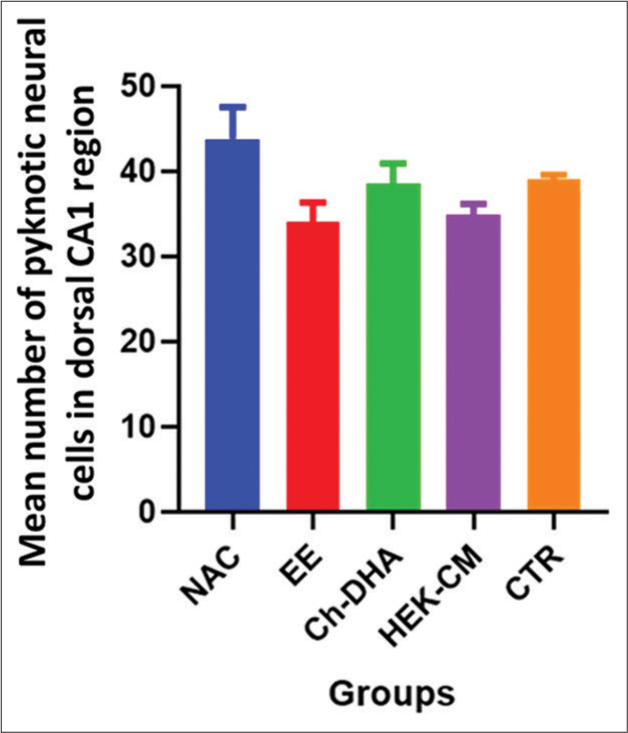
- Degenerating neural cell counts in dorsal hippocampal CA1 region of ageing mice. n = 6/group. Values represent the Mean ± SEM number of pyknotic neural cells in the CA1 region/HPF. A slightly lesser number of pyknotic/degenerating neural cells were found in EE-exposed and HEK-CM-treated mice brains when compared to the same in NAC mice. CA: Cornu ammonis, SEM: Standard error of the mean, EE: Enriched environment, HEK-CM: Human embryonic kidney stem cell conditioned media, Ch-DHA: Choline and docosahexaenoic acid, CTR: Clitoria ternatea root extract, NAC: Normal age-matched control.

- Representative photomicrographs of (30 μm thick) cross-sectional view of CA3 region of dorsal hippocampus from all ageing mice experimental groups. The black arrows represent pyknotic degenerating neural cells and the red arrows show lacunae related to shrinking/degenerating neural cells in ageing mice (Magnification: ×400). NAC: Normal age-matched control, HEK-CM: Human embryonic kidney stem cell conditioned media, Ch-DHA: Choline and docosahexaenoic acid, EE: Enriched environment, CTR: Clitoria ternatea root extract.
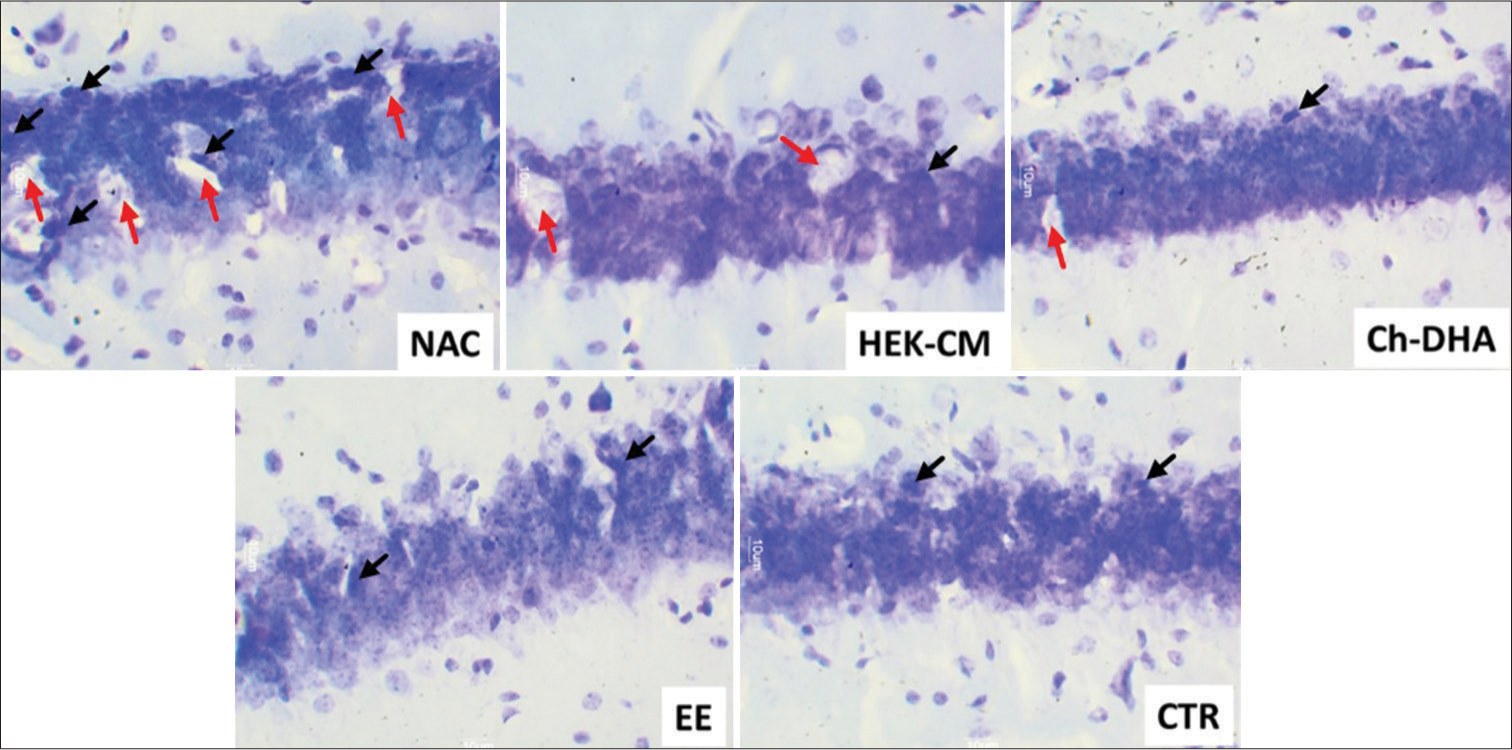
- Representative photomicrographs of (30 μm thick) cross-sectional view of CA2 region of dorsal hippocampus from all ageing mice experimental groups. The black arrows represent pyknotic degenerating neural cells and the red arrows show lacunae related to shrinking/degenerating neural cells in ageing mice (Magnification: ×400). NAC: Normal age-matched control, HEK-CM: Human embryonic kidney stem cell conditioned media, Ch-DHA: Choline and docosahexaenoic acid, EE: Enriched environment, CTR: Clitoria ternatea root extract.
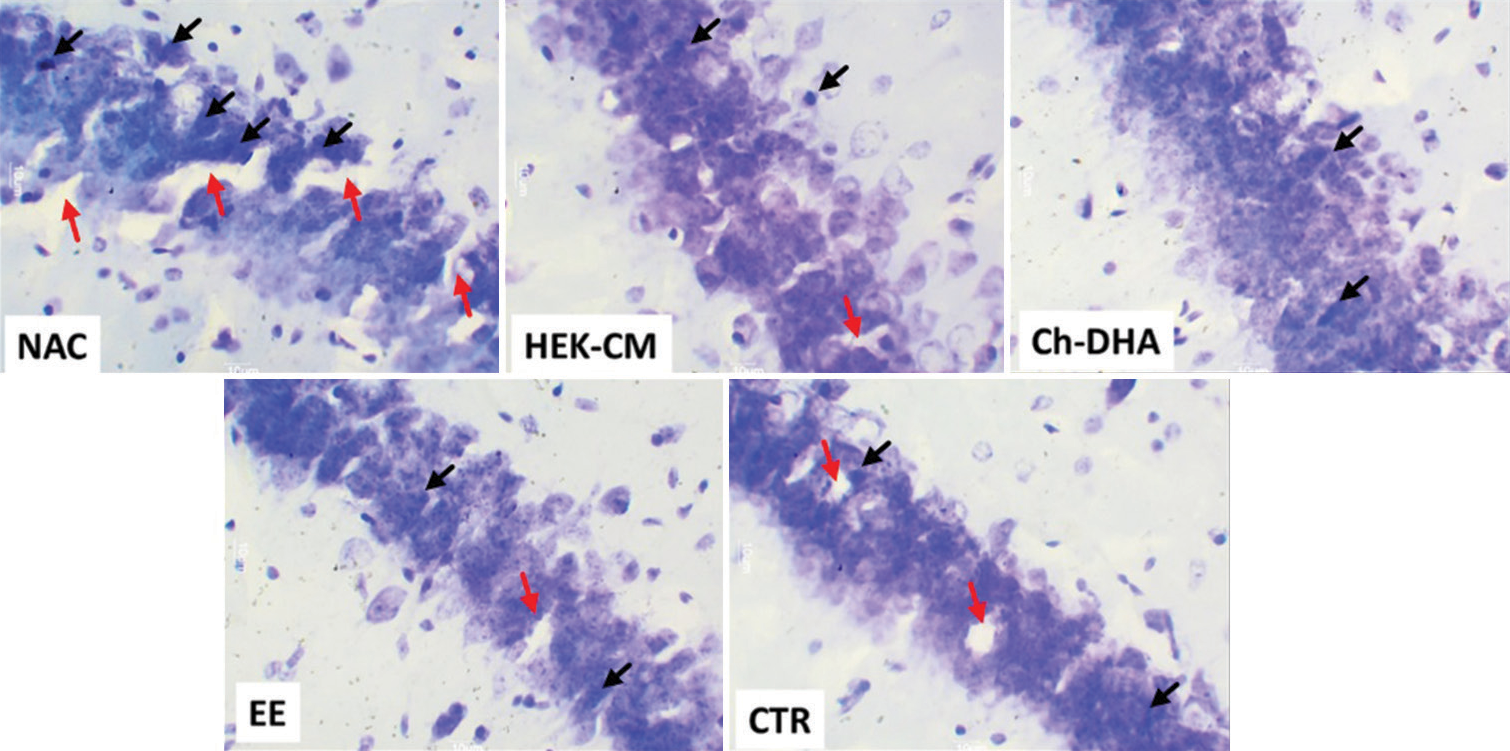
- Representative photomicrographs of (30 μm thick) cross-sectional view of CA1 region of dorsal hippocampus from all ageing mice experimental groups. The black arrows represent pyknotic degenerating neural cells and the red arrows show lacunae related to shrinking/degenerating neural cells in ageing mice (Magnification: ×400). NAC: Normal age-matched control, HEK-CM: Human embryonic kidney stem cell conditioned media, Ch-DHA: Choline and docosahexaenoic acid, EE: Enriched environment, CTR: Clitoria ternatea root extract.
DISCUSSION
The present study demonstrates an increase in the density of shrinking/degenerating pyknotic neural cells, accompanied by a heightened presence of lacunae, in the naturally ageing dorsal hippocampal subregions of mice. Strikingly, the use of preventive interventional strategies such as treatment with HEK-CM, Ch-DHA supplementation, exposure to EE and administration of CTR has been observed to mitigate these degenerative changes in hippocampal neural cells varyingly in the different subregions. Specifically, when the mean number of pyknotic neural cells was counted in hippocampal sections, it was found to be significantly lesser with lesser lacunae in the CA3, CA2 and CA4 sub-regions of the hippocampus, especially in mice groups treated with HEKCM, those supplemented Ch-DHA or exposed to EE and those administered CTR respectively, when compared to the same in age-matched NAC mice. Whereas a slightly lower number of pyknotic neural cells was observed in the CA1 sub-region of the hippocampus in EE and HEK-CM treated mice when compared to the same in age-matched NAC mice.
Previous research studies have shown that proliferation and mitosis of progenitor cells in the ageing mice hippocampus can be affected by choline availability during embryonic development. In comparison to the choline-supplemented groups, the number of apoptotic cells in the entire hippocampus area was more than 100% greater in the choline-deficient animals.[18] The availability of choline is one of the survival factors that regulate neuronal apoptosis.[19-22] Studies in developing mouse brains show that apoptosis in the foetal hippocampus was increased in infants whose mothers consumed low-choline diets, whereas it was lowered in infants whose mothers consumed high-choline diets.[18,23,24] Studies also show that omega-3 polyunsaturated fatty acid (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) supplementation enhances hippocampal functionality in aged mice. EPA and DHA supplementation increases hippocampal neuronal density and decreases apoptosis in the CA1 and CA3 regions in 19-month-old mice.[25] Choline deficient PEMT-/- mice supplemented DHA showed a decreased apoptosis in the foetal hippocampus.[26] Combined supplementation of Ch-DHA is known to reduce hippocampal neural cell degeneration and memory deficits. In high-fat diet-induced postnatal obese rats, Ch-DHA supplementation increased the number of surviving neural cells in the CA1 and CA3 sub-regions of the hippocampus compared to the same in age-matched obese rats.[27] Combined Ch-DHA supplementation in a rat with chronic cerebral hypoperfusion-induced brain injury has prevented neural degeneration and enhanced neuronal survival rate and density in the CA1 to CA4 regions.[28] Few animal studies show the beneficial effect of HEK-CM in reversing KA-induced hippocampal damage and protecting hippocampal neurons. KA-lesioned mice infused with HEK-CM had significantly protected CA1, CA3 and dentate hilus neurons against KA-induced neurodegeneration.[29] According to previous studies, EE exposure causes reduced spontaneous apoptotic cell death and seizure-induced damage in CA1, CA3 and dentate hilar neurons in the rat hippocampus by 45%.[30] In a rat model with stereotaxic mediated kainate stress to the hippocampus, CTR therapy showed increased hippocampus cellularity and improved memory performance.[31] These findings indicate that these strategies have the potential to attenuate age-associated hippocampal neural cell damage and enhance cognitive abilities to some extent. Plausibly, all the preventive treatment strategies used in the present study mitigate age-associated atrophy of the hippocampal neural cells overall, but with differential effects in its subregions. Such region-specific information may be useful for selecting the best therapeutic approach to modulate age-related hippocampal neural cell alteration in normal ageing.
In our previous research, we assessed spatial learning and cognition and discovered that these treatment approaches possess greater potential for improving cognitive functions in normal-ageing mice. We found that supplementations of Ch-DHA and HEK-CM treatment strategies had a higher potential (~20–30%) for enhancing spatial learning, memory and cognition, whereas exposure to EE enhanced only short-term memory.[32] Radulescu et al. define that ageing has an impact on cognitive decline because of alteration in synaptic plasticities and impaired Ca2+ signalling mechanisms.[33] According to a prior study, cognitive changes in both young and old rats were associated with modifications in dendritic structure and synaptic markers, as well as variations in the levels of neurochemicals such as brain-derived neurotrophic factor, nucleoporin 62 and the autophagy protein light chain 3.[34] These differences in the molecular correlates of cognitive function emphasise the need for further research focusing on developing effective strategies for rejuvenating the ageing brain.
CONCLUSION
Our study suggests that degeneration of neural cells in the dorsal hippocampal CA3, CA2 and CA1 subregions of normal ageing mice can be more effectively mitigated through supplementation of Ch-DHA and treatment with HEK-CM compared to exposure to EE or supplementation with CTR. However, it is worth noting that all interventions, including EE and CTR supplementation, offer some level of neuroprotection, particularly in CA3 hippocampal neural cells. This is the first study providing evidence for the comparative efficacy of these preventive interventional strategies in mitigating hippocampal neural cell degeneration during normal ageing in mice.
Acknowledgement
The authors would like to thank the Manipal Academy of Higher Education for providing all the infrastructure and support needed for this study.
Ethical approval
The research/study was approved by the Institutional Review Board at Kasturba Medical College, MAHE, Manipal, number IAEC/KMC/51/2022, dated 26/03/2022.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Neuropsychology of cognitive ageing, minimal cognitive impairment, Alzheimer's disease, and vascular cognitive impairment. Eur J Pharmacol. 2004;490:83-6.
- [CrossRef] [PubMed] [Google Scholar]
- What cognitive changes can be expected with normal ageing? Aust N Z J Psychiatry. 2001;35:768-75.
- [CrossRef] [PubMed] [Google Scholar]
- The human hippocampus: Functional anatomy, vascularization and serial sections with MRI. (4th ed). New York: Springer-Verlag; 2013.
- [CrossRef] [PubMed] [Google Scholar]
- A behavioral assessment of hippocampal function based on a subregional analysis. Rev Neurosci. 2004;15:333-51.
- [CrossRef] [PubMed] [Google Scholar]
- Involvement of hippocampal synaptic plasticity in age-related memory decline. Brain Res Rev. 1999;30:236-49.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of aging and stress on hippocampal structure and function. Metabolism. 2003;52(10 Suppl 2):17-21.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001;22:581-94.
- [CrossRef] [PubMed] [Google Scholar]
- Volume and number of neurons of the human hippocampal formation in normal aging and Alzheimer's disease. J Comp Neurol. 1997;379:482-94.
- [CrossRef] [Google Scholar]
- Hippocampal aging in rats: A morphometric study of multiple variables in semithin sections. Neurobiol Aging. 1981;2:265-75.
- [CrossRef] [PubMed] [Google Scholar]
- Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027-33.
- [CrossRef] [PubMed] [Google Scholar]
- Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol Aging. 2008;29:129-47.
- [CrossRef] [PubMed] [Google Scholar]
- Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. J Neurosci. 1996;16:4491-500.
- [CrossRef] [PubMed] [Google Scholar]
- Regionally specific loss of neurons in the aging human hippocampus. Neurobiol Aging. 1993;14:287-93.
- [CrossRef] [PubMed] [Google Scholar]
- Neurobiological bases of age-related cognitive decline in the rhesus monkey. J Neuropathol Exp Neurol. 1996;55:861-74.
- [CrossRef] [PubMed] [Google Scholar]
- Conservation of neuron number and size in entorhinal cortex layers II, III, and V/VI of aged primates. J Comp Neurol. 2000;422:396-401.
- [CrossRef] [PubMed] [Google Scholar]
- Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc Natl Acad Sci U S A. 1996;93:9926-30.
- [CrossRef] [PubMed] [Google Scholar]
- Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7.
- [CrossRef] [PubMed] [Google Scholar]
- Choline availability during embryonic development alters progenitor cell mitosis in developing mouse hippocampus. J Nutr. 2003;133:3614-8.
- [CrossRef] [PubMed] [Google Scholar]
- Apoptosis is induced by choline deficiency in fetal brain and in PC12 cells. Brain Res Dev Brain Res. 1997;101:9-16.
- [CrossRef] [PubMed] [Google Scholar]
- Choline deficiency induces apoptosis in primary cultures of fetal neurons. FASEB J. 2001;15:1704-10.
- [CrossRef] [PubMed] [Google Scholar]
- Choline deficiency-induced apoptosis in PC12 cells is associated with diminished membrane phosphatidylcholine and sphingomyelin, accumulation of ceramide and diacylglycerol, and activation of a caspase. FASEB J. 1999;13:135-42.
- [CrossRef] [PubMed] [Google Scholar]
- Opposing regulation of choline deficiency-induced apoptosis by p53 and nuclear factor κB. J Biol Chem. 2001;276:P41197-204.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal dietary choline availability alters mitosis, apoptosis and the localization of TOAD-64 protein in the developing fetal rat septum. Dev Brain Res. 1999;115:123-9.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal choline availability alters the localization of p15Ink4B and p27Kip1 cyclin-dependent kinase inhibitors in the developing fetal rat brain hippocampus. Dev Neurosci. 2001;23:100-6.
- [CrossRef] [PubMed] [Google Scholar]
- n-3 polyunsaturated fatty acids supplementation enhances hippocampal functionality in aged mice. Front Aging Neurosci. 2014;6:220.
- [CrossRef] [PubMed] [Google Scholar]
- Dietary docosahexaenoic acid supplementation modulates hippocampal development in the pemt-/-mouse. J Biol Chem. 2010;285:1008-15.
- [CrossRef] [PubMed] [Google Scholar]
- Hippocampal neural cell degeneration and memory deficit in high-fat diet-induced postnatal obese rats-exploring the comparable benefits of choline and DHA or environmental enrichment. Int J Neurosci. 2021;131:1066-77.
- [CrossRef] [PubMed] [Google Scholar]
- Prophylactic combined supplementation of choline and docosahexaenoic acid attenuates vascular cognitive impairment and preserves hippocampal cell viability in rat model of chronic cerebral hypoperfusion ischemic brain injury. Int J Basic Clin Pharmacol. 2015;4:522-30.
- [CrossRef] [Google Scholar]
- Infusion of human embryonic kidney cell line conditioned medium reverses kainic acid induced hippocampal damage in mice. Cytotherapy. 2014;16:1760-70.
- [CrossRef] [PubMed] [Google Scholar]
- Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat Med. 1999;5:448-53.
- [CrossRef] [PubMed] [Google Scholar]
- Age dependent neuroprotective effects of medhya rasayana prepared from Clitoria ternatea Linn, in stress induced rat brain. J Ethnopharmacol. 2017;197:173-83.
- [CrossRef] [PubMed] [Google Scholar]
- Exploring potential strategies to enhance memory and cognition in aging mice. F1000Res. 2023;12:141.
- [CrossRef] [Google Scholar]
- The aging mouse brain: Cognition, connectivity and calcium. Cell Calcium. 2021;94:102358.
- [CrossRef] [PubMed] [Google Scholar]
- Structural and molecular correlates of cognitive aging in the rat. Sci Rep. 2019;9:2005.
- [CrossRef] [PubMed] [Google Scholar]







