Translate this page into:
Role of an enriched environment in ameliorating early life stress-induced changes in structure and functions of hippocampus and amygdala in rats
*Corresponding author: T. R. Laxmi, Department of Neurophysiology, National Institute of Mental Health and Neurosciences (NIMHANS), Bengaluru - 560029, Karnataka, India. laxmi.nimhans@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Khokhar SK, Kambali M, Mussavira S, Bindhu OS, Laxmi TR. Role of an enriched environment in ameliorating early life stress-induced changes in structure and functions of hippocampus and amygdala in rats. Indian J Physiol Pharmacol 2022;66:16-28.
Abstract
Objectives:
This study aimed to understand whether an enriched environment (EE) in adulthood benefits in mitigating the early life stress-induced changes in the structure and functions of the hippocampus and amygdala.
Materials and Methods:
Male Wistar rats were exposed daily for 6 h to early maternal separation and isolation (MS) stress from postnatal days (PND) 4–14 and later at PND 60–70 days subjected to EE, while, the normal control (NC) rats were not subjected to stress but reared with the mother under standard housing conditions. The effects of MS and EE on adulthood behaviour were not subjected to stress but assessed by measuring the ambulatory, repetitive and anxiety-like behaviour. The study has also done the plasma corticosterone concentrations. The dendritic remodelling in the amygdala and hippocampus was assessed using the Golgi cox staining approach. Finally, the present study compared the reactive oxygen species-induced lipid peroxidation and total antioxidant capacity in MS rats as an indirect measure of oxidative stress to study the impact of MS stress on the limbic circuit and peripheral organs.
Results:
MS rats showed increased anxiety and lower plasma corticosterone levels. The pyramidal neurons’ dendritic plasticity displayed a different pattern, with shrinkage in the CA1 hippocampal neurons and hypertrophy in the amygdala’s primary neurons. Variations in antioxidant activity and peroxidation observed in NC to MS across tissues indicate the occurrence and management of oxidative stress in MS. The 10 days of EE in young adulthood helped to reduce MS stress-induced structural abnormalities in hippocampal and amygdala pyramidal neurons, as well as anxiety and plasma corticosterone levels.
Conclusion:
These findings together indicate that exposure to adverse experiences may cause harmful effects on brain plasticity and behaviour in young adulthood. Exposure to EE may be beneficial in reducing the early life stress-induced pathophysiology later in life.
Keywords
Early life stress
Repetitive behaviour
Anxiety
Corticosterone
Neural plasticity
INTRODUCTION
John Locke (1632–1704) said that ‘the mind is a blank slate (tabula rasa) at birth, ready to be written on by experience.’ These experiences built up entirely from birth, through adolescence to young adulthood inducing changes in the structure and functions of neurons,[1] resulting in changes in behaviour. We previously found that an enriched environment (EE) was beneficial, showing early fear extinction, changed network activity between infralimbic regions of the medial prefrontal cortex, amygdala, and hippocampus[2], and increased synaptic plasticity.[3]
While, diminished fear memory and extinction was observed in animals exposed to impoverished environments such as maternal separation[4] and isolation stress at critical developmental time window[5,6] are associated with an impaired network activity in the same fear circuit.[7] Further studies have shown that anxiety-like behaviour was correlated with altered oxidative stress following either chronic social isolation stress or maternal separation stress.[8-10]
These evidence indicates that the brain has the remarkable ability to undergo plasticity changes in response to environmental stimuli such as nutrition,[11] EE[2], and a stressed environment.[7,12,13] We previously showed that 10 days of EE before the fear conditioning and subsequently after the extinction training can be more effective in the extinction of fear memory in rats reared in standard housing conditions.[2] In clinical studies, exercise has been shown to increase brain volume in areas implicated in processing, improve cognition in children with cerebral palsy and enhance phonemic skills in school children with reading difficulties,[14] as well as difficulties with external/ internal temperature modulation.[15] A recent study from our laboratory has further found that long-term exposure to a combinational paradigm consisted of EE, physical exercise and diet-rich nutrition can reverse the ibotenic acid lesioning of the ventral subiculum on spatial memory deficit and neurogenesis in the hippocampus.[16] The above studies, thus, confirm that an EE can directly impact brain plasticity, bolstering the theory that EE influences brain structure and function throughout an organism’s lifespan.
However, there are only few studies have examined the impact of EE in brain growth in stressful conditions. One of the critical steps is to define normal brain growth and how EE can enhance cognition. The changes in neuronal morphology and functional connectivity in the limbic system, notably in the hippocampus, amygdala, and medial prefrontal cortex, which regulates learning, memory, and mood, could be the biological basis for these effects. A plethora of evidence in the literature indicates that EE in adulthood promotes neurogenesis, maturation, and synaptogenesis in hippocampal formation, which is crucial for learning and memory.[17] The BDNF, in turn, regulates the adult hippocampal neurogenesis and synaptogenesis, both of which are important for learning and memory.[18]
Separation and isolation stress (MS) from their mothers during the stress hyporesponsive period (SHRP) which is similar to the children under five are highly prevalent to anxiety disorders later in life. Although the early childhood environment is vital, policies are to be in place to minimise the negative experiences during this critical developmental period on adult emotionality behaviour.[19] Childhood stress is prevalent in a progressive country like India. In particular, the SHRP is a critical period for the rat pups that shapes the brain and behaviour in young adulthood[20,21] which is basically because of the reduced adrenal gland’s response to stress.[22] Any adverse experiences during this period can cause long-lasting changes in the brain and behaviour.[23] As a result, SHRP becomes an important period, wherein adulthood behaviour is shaped at a very early period of life. Accordingly, the present study has examined the effect of 10 days of EE exposure at young adulthood on anxiety, repetitive and ambulatory behaviour, basal corticosterone, total antioxidant capacity (TAC) in the fear circuit. The dendritic plasticity in the pyramidal neurons of the hippocampus and amygdala was examined to see whether exposure to an EE impacts the reversal of anxiety and ambulatory functions. Finally, we also evaluated the biochemical changes to study how MS stress affects antioxidant capacity and lipid peroxidation (LP) in different brain regions and other tissues.
MATERIALS AND METHODS
Subjects
Pregnant Wistar rats were obtained from the Central Animal Research Facility, at NIMHANS, Bengaluru on a gestational day of approximately 18–19. Dams were individually housed in propylene cages with husk bedding on a 12:12 h light-dark cycle with free access to water and food ad libitum. All the procedures were approved by the Institutional Animals Ethics Committee and maximum care was taken to minimise the pain and discomfort to the experimental animals during the procedure.
The rats were randomly divided into four groups and kept in two different housing conditions – standard housing and EE conditions. The first group, normal control (NC), was reared with a mother in a standard house condition without exposure to MS procedure. The second group, maternal separation and isolation stress (MS), was exposed to MS stress procedures during SHRP. The third group, NC-EE, was reared with the mother during the entire pre-weaning period and exposed to an EE condition during P60-P70. The fourth group, MS-EE group, was exposed to MS stress during SHRP and exposed to an EE condition during P60-P70.
MS procedure
Maternal separation and isolation (MS) stress procedure was carried out for 10 days during the postnatal day (PND) 4–14. During this SHRP, rat pups were separated from their mother and subsequently isolated from their littermates. Isolation of pups was carried out in a partitioned cage having six compartments with a little husk in each cage. The stress protocol was carried out for 6 h daily, from 9.00 AM to 3.00 PM. After this MS procedure, rat pups were reunited with their mothers. Rat pups were weaned from the mother on PND 22. All the behavioural and Golgi studies were carried out when the rat was on 2–3 months of age.
EE conditions
EE rats were placed, in groups of 7–10, in a special cage measuring 102 cm × 64 cm × 61 cm. The EE chamber was with various objects such as ladders, tunnels, wooden pieces of different shapes and sizes, and interchangeable toys such as balls and building blocks. To provide opportunities for both sensory and physical stimulation which were rearranged every day and changed into different objects on alternate days for novelty.[2] EE conditions were daily for 6 h/day for 10 days during P60– P70. A different cohort of rats was used for EE group per se and all testing was carried out during the light phase in 2–3-month old rats.
Behavioural assay
[Figure 1] is depicting the timeline of the entire study. All the behavioural assays were performed on PND 71–73, while the rat brain from all four groups was processed for the Golgicox staining procedure on PND 71. A different cohort was used for the biochemical assay on PND 71.
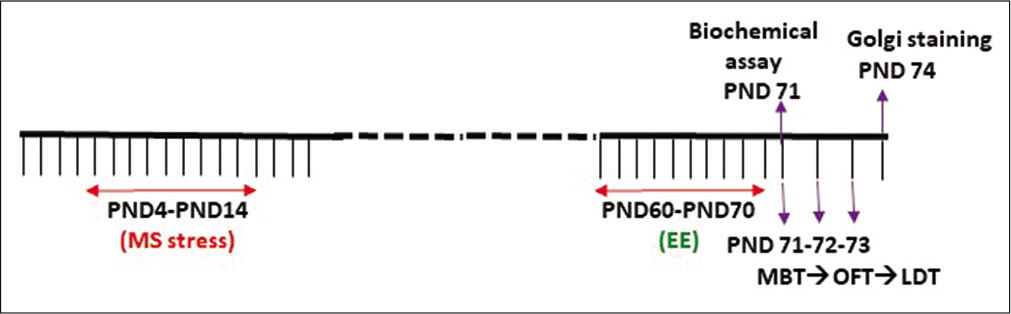
- Experimental design depicting the time line of the study.
Light dark test
This etiological model of anxiety test measures the unconditional anxiety-like behaviour that is based on an approach/avoidance conflict between the drive to explore novel areas and an aversion to brightly lit and open spaces. The anxiety test was conducted on PND 71 in the Coulbourn Habitest rat shuttle cage (Coulbourn Instruments Inc, USA). The shuttle cage consisted of two compartments of equal size (26 × 26 cm) separated by a sliding guillotine door (8 × 8 cm). The experimental design was based on our previous study.[24] The rat was placed in a brightly lit environment (approximately 300 Lux) and the guillotine door was opened within 10 s after placing the rat. The rat behaviour was observed for 10 min in the light-dark chamber and the entire experiment was videotaped for offline analysis. After 10 min of the exploration, both the chambers were cleaned with 70% ethanol. The data were analysed for the latency to enter the dark chamber, total time spent in the dark chamber, and the number of transitions it makes during the given period.
Marble burying test
The rectangular glass chamber (24 × 12 × 11.75 cm) was filled with husk for up to 4.5 cm in height. The test was carried out 24 h after the light-dark test (PND72). The husk was tapped down to make it a flat and even surface. The regular pattern of glass marbles (total marbles = 18) was arranged 3 cm apart from each other and spaced evenly. Each animal was allowed to explore the chamber for 10 min. Rat’s burying behaviour, up to 2/3rd depth, was observed during this period. The walls of the chamber were cleaned with 70% alcohol after each experiment. In addition, these marbles were cleaned with 70% alcohol after each experiment. The number of marbles buried (>50% marble covered by husk) and unburied (<50% marble unburied by husk) were counted manually, as an index of anxiety and repetitive behaviour.
Open field test
Animals were placed in an open rectangular Plexiglas arena (120 × 80 × 40 cm) with a trial duration of 10 min. The floor was divided into periphery and centre. The light was focused in the centre with 300lux and with minimum light (up to 10lux) at the corner and periphery. The rat was exposed to an open field arena on PND 73 and allowed to explore freely for 10 min. Time spent in the centre of the field was quantified as the reciprocal proxy of the anxiety. The total distance travelled during the trial was also quantified as a measure of locomotion. Experiments were recorded in a video camera from a suitable distance to cover the entire open field.
Golgi study
The morphological study was also carried out to explain the neural plasticity in the areas which are critical for spatial learning and memory – the medial prefrontal cortex, hippocampus, and amygdala.
Golgi-Cox staining to study the dendritic arborisation tissue preparation
To study the dendritic arborisation of the hippocampus and amygdala, Golgi-Cox staining was performed. A fresh batch of rats at the age of PND 71 was used to evaluate the impact of MS stress and EE on dendritic morphology. Rats from all four groups were sacrificed. The brain was removed, washed with distilled water followed rinsed in a freshly prepared Golgi–Cox solution.[25] The brain was cut into two 5 mm thick coronal blocks and placed in Golgi–Cox solution in amber bottles at 37°C for 24 h. Later, these brains were kept in dark conditions at room temperature (RT) for 8–10 days. After fixation in Golgi–Cox solution, 170 µm thick coronal sections were taken onto gelatine coated slides and these sections were processed with sodium carbonate solution (10%) followed by dehydration using a gradient of ethanol in order 70% (10 min), 80% (2 min), 90% (2 min) and 100% (1–2 min). The slides were flooded with cedar oil (Sigma-Aldrich, #96090) overnight. On the next day, the excess cedar oil was drained away and the slides were further cleared with xylene. The slides were mounted with DPX and coverslips were placed. Each slide was coded and the code was broken just before plotting the graph and for statistical analysis.
Analysis of dendritic arborisation of pyramidal neurons
The neuronal reconstruction was accomplished using NeuroLucida (Micro-brightfield Inc., Colchester, USA) under ×40 objective of the Olympus BX61 Microscope (Olympus Microscopes, Japan). The pyramidal neurons from the basolateral amygdala (LA) and CA1 area of the hippocampus were considered in the study. The neuronal reconstruction was carried out for pyramidal neurons of all the above regions with a minimum of ten neurons from each area per rat brain. Both hemispheres were considered separately for the study.
Using NeuroLucida explorer (Micro-brightfield Inc., Colchester, USA), the Sholl analysis was carried out to calculate the dendritic length, the number of branch points (nodes), and the number of intersections made by the dendrite on the concentric circles drawn by considering the centre of soma as a reference point. Concentric circles were of 10 µm radius and values were added for each consecutive radial segment. The total length of 300 µm radial distance from the soma was considered for analysis in basal dendrites and 400 µm radial distances from the soma in apical dendrites. This helped to maintain uniformity and decrease bias.
Biochemical assay
Selected brain tissues (amygdala, prefrontal cortex, and hippocampus) and other tissues (adrenal gland, liver, spleen, and blood) were harvested from MS (n = 4), MS-EE (n = 4), and NC (n = 5) rat groups at PND 71. All solid tissue samples were macerated in chilled phosphate buffer using a pestle and mortar and placed in an ice bath. Blood was allowed to clot and serum was collected after centrifugation. The supernatant after centrifugation of tissue extracts and serum was used for TAC and LP assays.
LP assay
LP was measured by the FOX2 method where 200 µl tissue extract/serum reacts with 1.8 ml of FOX2 reagent (250 mM ammonium ferrous sulfate, 100 mM xylenol orange, 25 mM H2SO4, and 4nM butanol hydroxytoluene in 90% v/v methanol) at RT. Peroxides in samples cause oxidation of ferrous iron to ferric iron that reacts with xylenol orange to form a ferric-xylenol orange complex.[26,27] The absorbance of this complex was measured spectrophotometrically at 560 nm after a 30 min incubation at RT against a blank comprising only FOX2 solution prepared without ferrous sulphate. Sample peroxide content was thus determined using standards of H2O2 aliquots.
TAC
TAC was measured by the method given by Koracevic et al.[28] Briefly, 10 µl of tissue extract/serum, 490 µl of phosphate buffer (100 mM, pH 7.4), 500 µl of 10 mM sodium benzoate, 200 µl each of 2 mM Fe-EDTA complex, and 10 mM H2O2, were added. This reaction mixture was incubated at 37°C for 60 min and arrested with 1 ml 20% acetic acid. An internal blank was prepared for each sample by adding acetic acid to the reaction mixture before incubation. The Fe-EDTA complex reacts with H2O2 to form thiobarbituric acid reactive substances (TBARS). These TBARS were allowed to interact with 1 ml of thiobarbituric acid (0.8% w/v in 50 mM NaOH) for 10 min at 100°C. The coloured product thus formed was measured spectrophotometrically at 532nm. This colour development can be suppressed by antioxidants in the added tissue extract or serum, which defines its TAC.
Statistical analysis
The appropriate statistical analysis was carried out using GraphPad Prism Software (7.0 version) to compare between and within groups. The comparisons between groups and changes in dendritic arborisation in CA1 and LA at different length and intersections were made using two-way ANOVA followed by Bonferroni post hoc test. The behavioural assays between groups were compared using one-way ANOVA. The biochemical assays were compared between groups using Student’s t-test. P < 0.05 was considered significant.
RESULTS
Any adverse experience at the critical time window of the development causes changes in the brain and behaviour such as anxiety. An animal model of early life stress was developed by maternal separation and isolation stress at SHRP of the rat. In the present study, we used a rat model to show that 10 days of EE in adulthood could benefit maternally separated animals minimise anxiety-like behaviour. The stress model was validated at the start of the study by analysing anxiety-like behaviour.[6]
Anxiety-like behaviour
When rats were exposed to a brightly lit environment, they tend to explore the dark and safe environment. In the light-dark test, MS rats spent relatively more time in the dark compartment than NC rats [Figure 2a]. The MS rats having an exposure to EE showed reduced time in the dark compartment than MS with no EE exposure, which is statistically significant [Figure 2a] (t1,17 = 2.026, P < 0.05) indicating anxiolytic behaviour. However, there was no significant difference in time spent in the dark compartment between NC and NC-EE groups. In addition, we did not observe any significant differences in latency to enter the dark chamber (even though there is the trend of reduced latency) [Figure 2b] and the total number of transitions between compartments between groups [Figure 2c].
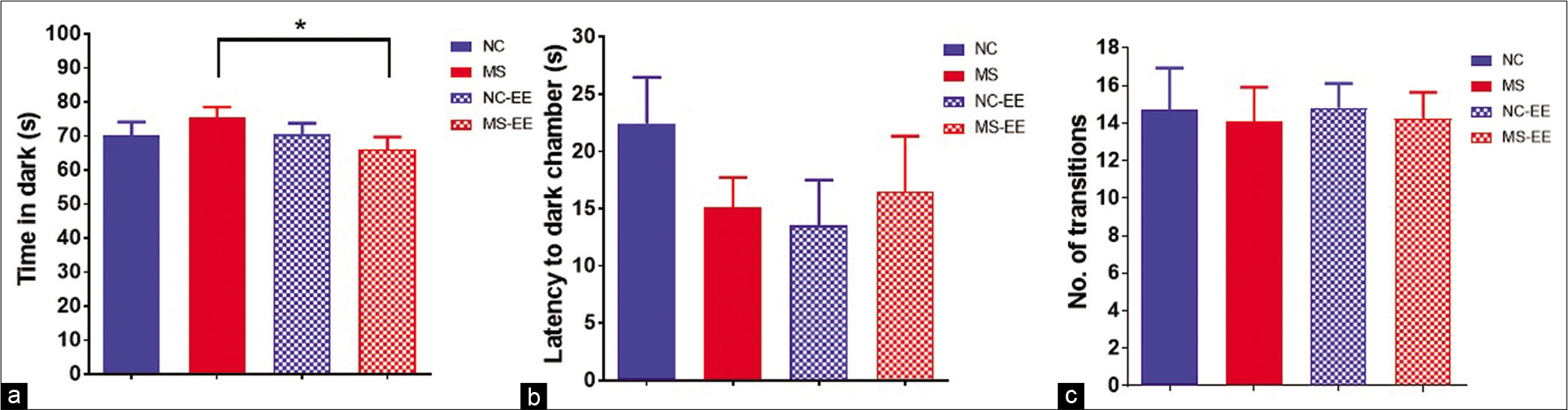
- Anxiety-like behaviour in rats in the light-dark test. (a) Time spent in dark compartment; (b) Latency to enter into dark compartment; (c) total number of transitions between light and dark compartments; data are represented in Mean ± SEM, Student’s t-test analysis, t1,17 = 2.029, *P < 0.05, normal control (NC) (n = 11), maternal separation and isolation stress (MS) (n = 11), NC-enriched environment (NC-EE) (n = 11) and MS-EE (n = 8).
Marble burying behaviour
The anxiety-like behaviour and repetitive behaviour were further tested using the marble-burying paradigm [Figure 3]. The results indicated that there was a significant difference (P < 0.001) between MS and NC groups showing MS rats buried a greater number of marbles when compared to NC rats. The statistical analysis using one-way ANOVA suggested a significant difference between the groups (F3,47=16.63, P < 0.0001). However, no significant differences in marble-burying behaviour were observed between NC-EE and MS-EE groups. While, between MS and MS-EE groups, MS-EE rats buried a lesser number of marbles when compared to MS rats which was statistically significant (P < 0.001). A statistically significant difference was also observed between NC-EE and MS groups (P < 0.001). Thus, these results indicate that EE in MS rats has a significant impact on ameliorating the stress-induced repetitive and anxiety-like behaviour.
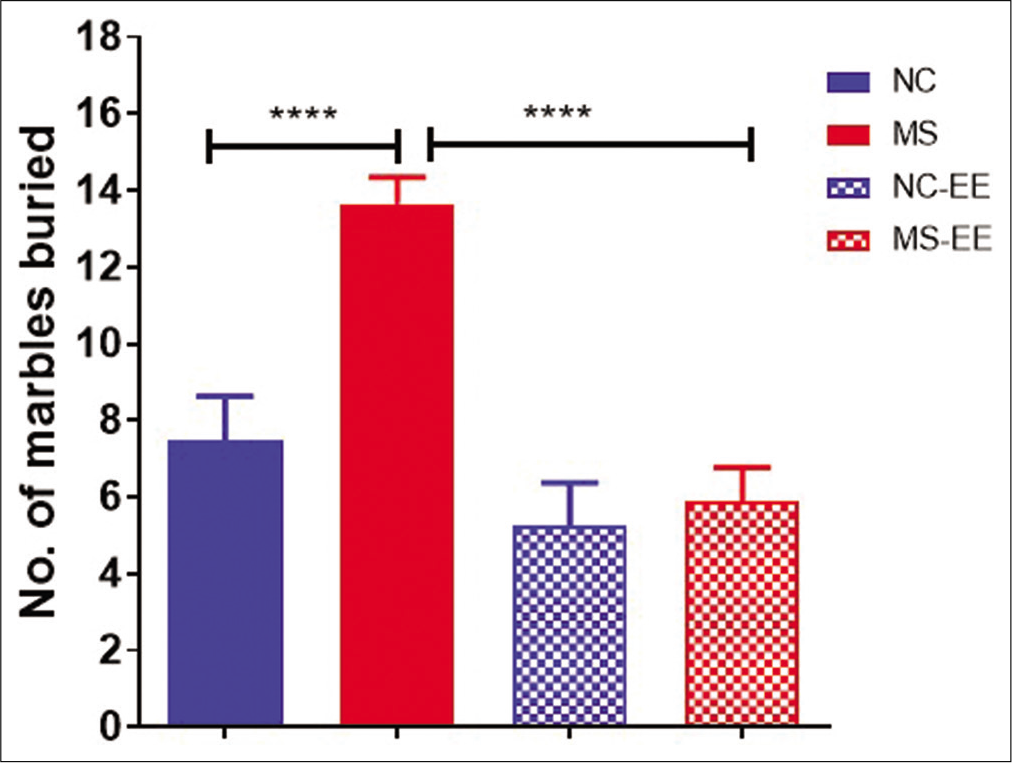
- Marble burying behaviour of rats after MS stress and EE exposure. Data are represented in mean ± SEM, One-way ANOVA analysis, F3,47=16.63, ****P < 0.0001, Normal control (NC) (n = 15), maternal separation and isolation stress (MS) (n = 17), NC-Enriched environment (NC-EE) (n = 11) and MS-EE (n = 8).
Open field test
The open-field test was used to evaluate both locomotor activity and anxiety-like behaviour in the rats when subjected to the open field. The results indicated that both MS and NC-EE rats showed increased ambulatory activity in the open arena. This was calculated based on the number of crossings in the open field. MS, MS-EE, and NC-EE rats were explored more in the peripheral zone when compared to NC rats [Figure 4a]. To quantify the anxiety-like behaviour, we have counted the number of entries into the central zone [Figure 4b] and also the time spent in the central zone [Figure 4c]. The results using one-way ANOVA indicated that both NC-EE and MS-EE rats entered the central zone more frequently than NC and MS animals which are statistically significant (F3,41 = 14.53; P < 0.0001). These two groups also spent more time in the central zone (F3,41 = 11.42; P < 0.0001) indicating EE had a beneficial effect in reducing the anxiety-like behaviour. In summary, the results indicated that EE to MS was beneficial in terms of reducing anxiety-like behaviour and ameliorating ambulatory behaviour.
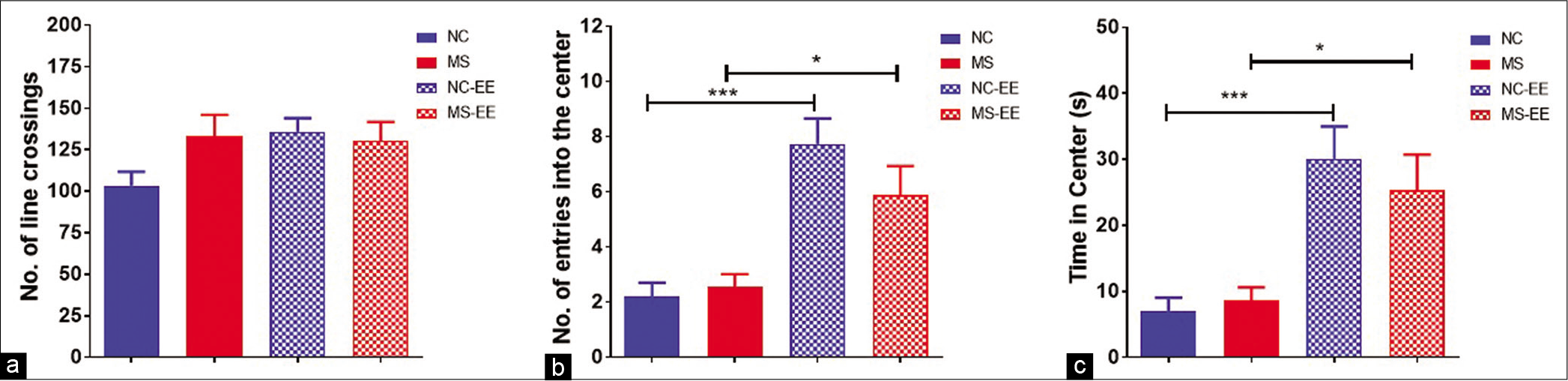
- Open field test. (a) Number of line crossings, (b) number of entries into the centre and (c) time spent in the centre; data are represented as mean ± SEM, One-way ANOVA analysis followed by Tukey’s multiple comparison test; ***P < 0.001, *P < 0.05; normal control (NC) (n = 15), maternal separation and isolation stress (MS) (n = 11), NC-enriched environment (NC-EE) (n = 11) and MS-EE (n = 8).
Corticosterone assay
In the present basal, corticosterone assay was performed in young adult rats. It was observed that MS rats showed reduced basal corticosterone as compared to NC group (P < 0.001) [Figure 5]. On EE exposure, it was observed that the basal corticosterone was partially increased in both NC-EE and MS-EE groups showing statistically no significant difference between NC-EE and MS-EE rats as well as with that NC group.
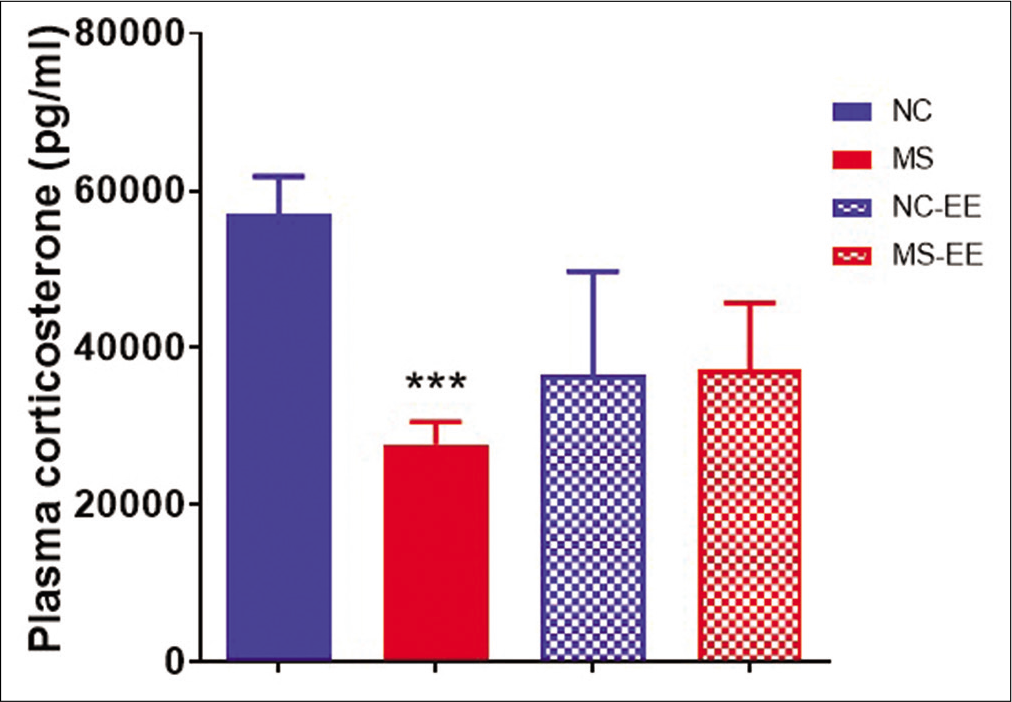
- Effect of MS stress and EE on plasma corticosterone levels. Data represented are in mean ± SEM, ***P < 0.001 when compared to NC. MS (maternal separation and isolation stress) (n = 5), NC (normal control) (n = 5), NC-EE (NC-enriched environment) (4) and MS-EE (n = 3).
Golgi study
Next, the morphological study was carried out to understand the neural plasticity in the areas which are critical for spatial and fear learning and memory – the hippocampus and amygdala. The pyramidal neurons of CA1 region of the hippocampus were characterised by long apical and basal dendrites having extensive arborisation, while, LA pyramidal neurons are shorter than CA1 pyramidal neurons. In the MS stress group, the apical dendrites of the CA1 hippocampus were shorter and LA neurons were longer and more dense as compared to controls. EE for 10 days in young adulthood was beneficial in reversing the MS stress-induced changes in CA1 and LA neurons.
Amelioration of MS stress-induced reduction in dendritic arborisation in a distal branch of pyramidal neurons of CA1 region of the hippocampus following EE
MS stress during SHRP led to a significant decrease in the total dendritic length of pyramidal neurons of CA1 subregion of the hippocampus as compared to NC CA1 pyramidal neurons (F3,3973 = 71.12; P < 0.0001) [Figure 6a]. The dendritic length of apical dendrites was more specifically reduced significantly at 60–80 µm distance from soma (P < 0.05); while a significant difference in the basal dendritic length was not observed between NC and MS groups. Similarly, the number of intersections was significantly reduced (P < 0.05) in apical dendrites of CA1 pyramidal neurons as compared to NC groups [Figure 6b].
Exposure to 10 days of EE had a beneficial effect on dendritic arborisation of CA1 hippocampal neurons showing restoration of MS stress-induced reductions in dendritic length of apical dendrites of pyramidal neurons. A significant difference (P < 0.05) was found between MS and MS-EE at 150–160 µm. Interestingly, the difference observed between NC and MS was continued even after EE exposure in adult life showing a significant difference between NC-EE and MSEE (P < 0.05) at 100–170 µm.
MS-EE group showed enhanced dendritic length at the proximal branch of apical dendrites as compared to NC group at 50– 90 µm. Interestingly, NC-EE group showed further enhancement in dendritic length from 140 to 170 µm as compared to NC (P < 0.05) group [Figure 6b]. Similarly, the number of dendritic intersections at 130-170µm was significantly more in NC-EE than NC (P < 0.05), while, they were significantly less in MS-EE than MS-EE at 130-160µm (P < 0.05) [Figure 6c].

- (a) Photomicrographs of Golgi-stained sections of CA1 pyramidal neurons from normal control (NC), maternal separation stress (MS), NC-enriched environment (NC-EE) and MS-EE rats showing the dendritic arborisation of pyramidal neurons of CA1 hippocampus. (b) Apical dendritic length of CA1 pyramidal neurons; (c) apical dendritic number of intersections of CA1 pyramidal neurons; (d) basal dendritic length of CA1 pyramidal neurons and (e) basal dendritic number of intersections of CA1 pyramidal neurons; bars represent mean ± SEM; NC = 33; MS = 34; NC-EE = 27 and MS-EE = 35 neurons from each group 10 subjects from CA1 area of hippocampus.
The basal dendrites, on the other hand, did not show a significant difference between NC and MS groups. Interestingly, as compared to NC and MS, the basal dendritic length of CA1 hippocampus was significantly increased (P < 0.05) in dendritic length and dendritic intersections of both NC and MS-EE, respectively, at distances of 80–170 µm from soma [Figures 6d and e]. MS-EE group showed enhanced dendritic length at the distal branch of basal dendrites with a significant difference between MS-EE and NC group from 100 to 170 µm. However, dendritic length from 50 to 100 µm did not get restored following EE. Interestingly, NC-EE group showed further enhancement in dendritic length from 110 to 150 µm as compared to NC (P < 0.05) group [Figure 6b].
Partial restoration of MS stress-induced hypertrophy of LA pyramidal neurons
EE was successful in ameliorating MS stress-induced hypertrophy of pyramidal neurons [Figure 7a] as that of NC and NC-EE (F3,3188 = 22.16; P < 0.001) [Figure 7b]. The enhancement of dendritic branches in the apical branch was not specific to proximal and distal dendrites for NC and MS groups; rather, it was an overall increase in the dendritic length of LA pyramidal neurons. Exposure to EE was beneficial for the partial restoration at a 140 µm distance from soma (P < 0.05) [Figure 7b]. A similar trend was observed with the total number of intersections in apical dendrites [Figure 7c].
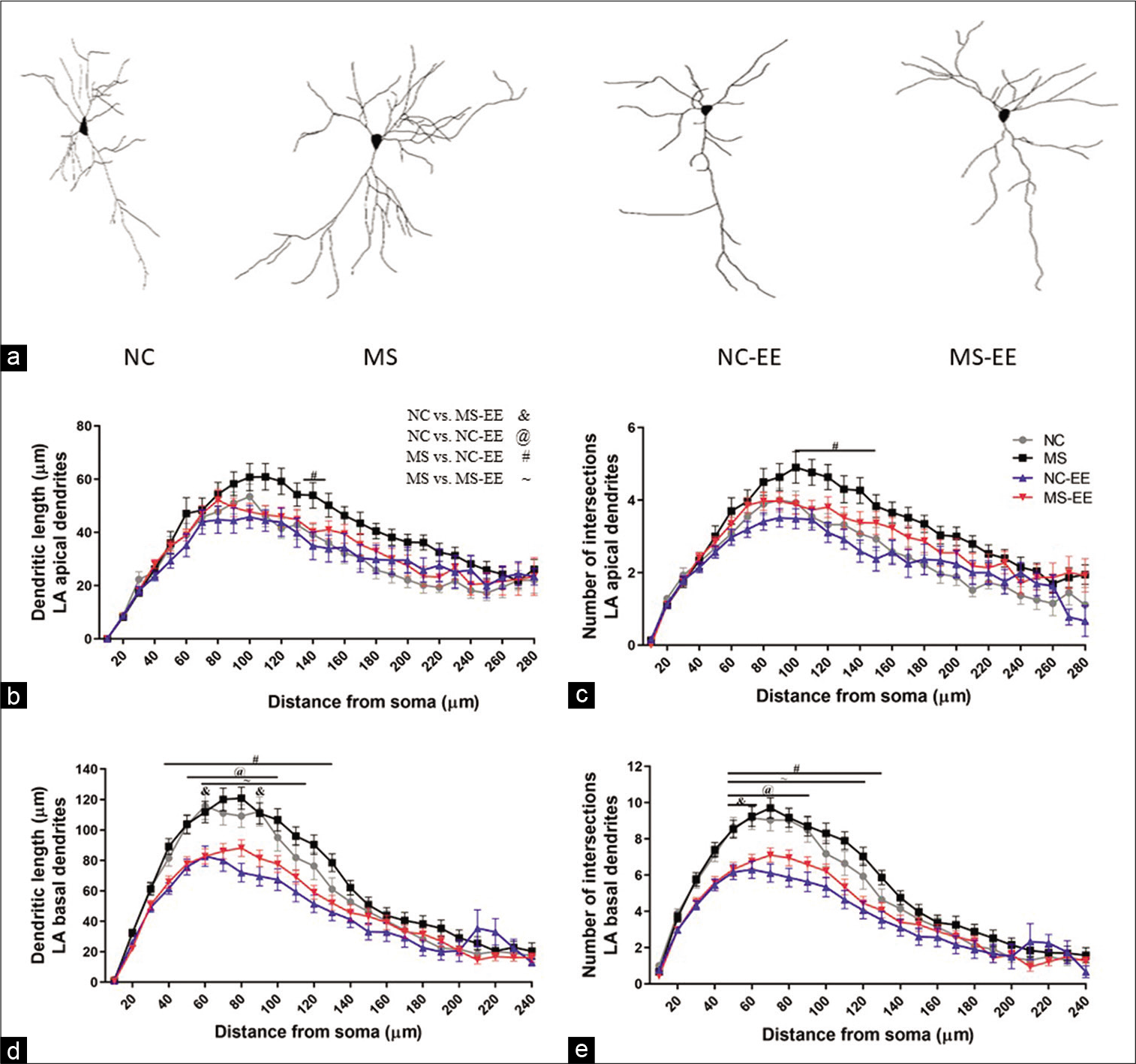
- (a) Photomicrographs of Golgi-stained brain sections from normal control (NC), maternal separation stress (MS), NC-enriched environment (NC-EE) and MS-EE rats showing the dendritic arborisation of pyramidal neurons of LA amygdala; (b) apical dendritic length of LA pyramidal neurons; (c) apical dendritic number of interaction of LA pyramidal neurons; (d) basal dendritic length of LA pyramidal neurons and (e) basal dendritic number of interaction of LA pyramidal neurons; bars represent mean ± SEM; NC = 28; MS = 30; NC-EE = 37 and MS-EE = 41 neurons from each group 10 subjects from LA of amygdala pyramidal neurons.
The significant changes were observed in basal dendritic intersections and dendritic length between NC-EE and MSEE groups as compared to respective controls [Figures 7d and e]. As compared to NC, NC-EE group showed reduced dendritic length at the proximal branch of basal dendrites of LA pyramidal neurons at 50-100µm (p<0.05) [Figure 7d]. Similarly, MS-EE group also showed reduced dendritic length at 60-120µm as compared to MS group (p<0.05) [Figure 7d]. A significant difference was observed with dendritic intersections between NC and NC-EE at 50–90µm (P < 0.05), as well as, a significant difference was observed between MS and MS-EE group 50–120µm (P < 0.05) as compared to MS group [Figure 7e]. Further, there was no significant difference between NC-EE and MS-EE group.
TAC and LP
The t-test analysis revealed that TAC in MS rats was significantly increased in spleen (P < 0.008) and liver (P < 0.003); while, no changes were observed in hippocampus, amygdala and PFC as compared to controls [Figure 8a]. On the other hand, LP was not changed in PFC, amygdala and hippocampus, but significant increase was found in adrenal gland of MS rats when compared to NC [Figure 8b].
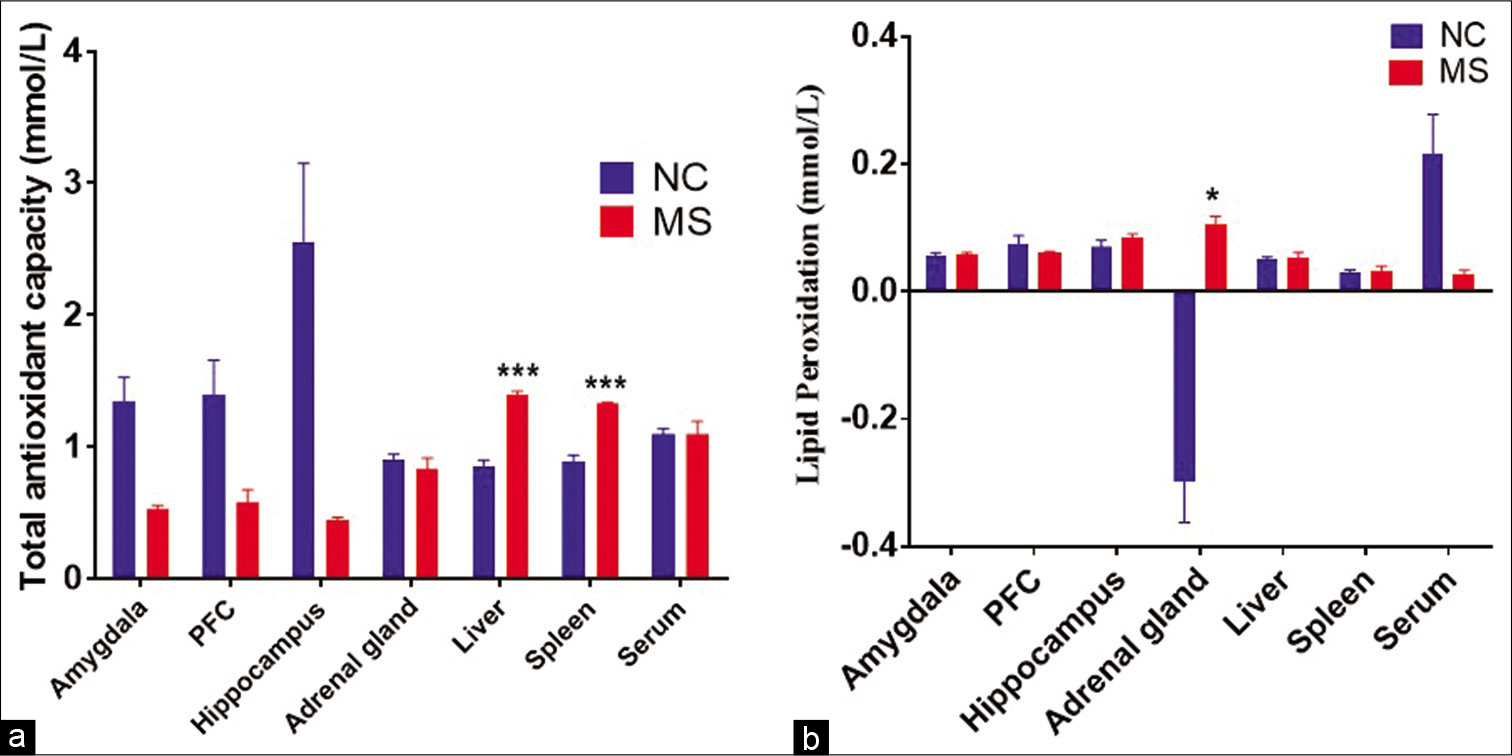
- The effect of MS stress on (a) total antioxidant capacity and (b) lipid peroxidation in different bran regions and organs. Data represented are in mean ± SEM. NC, Normal control, (n = 5); MS, maternal separation (n = 4); PFC, prefrontal cortex and TAC, total antioxidant capacity, *P < 0.05, ***P < 0.001.
DISCUSSION
The present study has demonstrated the long-term impact of chronic MS stress during SHRP and subsequent exposure to 10 days of EE at the young adulthood stage on brain and behaviour. In the present study, we have shown that EE was beneficial in ameliorating MS stress-induced changes in dendritic arborisation of the hippocampus and amygdalar pyramidal neurons, affective behaviour, stress hormones, and TAC.
The critical findings from the present study are: (1) Both distal and proximal dendrites of CA1 and LA pyramidal neurons were affected by MS stress. MS stress has contracted the proximal apical dendrites of CA1 pyramidal neurons. EE was beneficial in restoring the contracted apical dendrites at the distal but not at proximal branches. The basal CA1 dendrites, on the other hand, did not get affected by MS stress. However, EE was beneficial for CA1 neurons by remodelling the dendritic arborisation at proximal and distal dendrites. The dendritic remodelling for LA pyramidal neurons was observed at the basal dendrites than at apical dendrites following EE exposure. (2) MS stress-induced changes in anxiety, ambulatory and repetitive behaviour were restored following exposure to EE. (3) The study further observed partial restoration of basal plasma corticosterone following EE in young adulthood. (4) MS stress did not significantly impact TAC and LP in the amygdala, hippocampus, and prefrontal cortex.
In the developing brain, SHRP, per se, the hypothalamus-pituitary-adrenal (HPA) axis responds and releases hormones poorly to stressful stimuli.[29] Any adverse experience during such a critical time window of development (SHRP) may cause long-lasting changes in affective behaviour later in life. Even though the exact neurophysiological mechanisms following MS stress are not known, there is evidence indicating that stress causes maladaptive changes in the HPA axis and also on feedback mechanisms.[22,30] In contrast to the previous study,[22] the present study has observed reduced basal plasma corticosterone in MS rats which could be a consequence of sustained activation of the HPA axis, due to chronic MS stress. As a result of the maladaptive HPA axis, there may be a significant change in glucocorticoid release in young MS adult rats. Thus, it may be implicated in the increased risk for anxiety disorders in young adulthood.[31] The differences in the basal corticosterone observed in our study could be due to two types of stress given to rat pups during SHRP – maternal separation and isolation stress. The reduced corticosterone and gradual recovery following EE exposure, indicating a MS stress at SHRP, severely disrupted the HPA system, which was not restored entirely even after 10 days of EE in young adulthood. In support of this, studies have shown that physical exercises enhance the adaptive properties of the HPA axis by altering the adrenal gland sensitivity for the release of glucocorticoids[32,33] to maintain homeostasis.
The heightened anxiety-like behaviour of MS rats in a light-dark box is consistent with our previous findings.[5] Similarly, the study has also found an increased marble-burying behaviour and an increased ambulatory behaviour in the open field paradigm of MS rats indicating that anxiety is a widespread phenomenon in animals exposed to early life stress. The 10-days of EE programme was proven to effectively lower the MS stress-induced elevated anxiety and reduce repetitive behaviour. EE was also advantageous for both NC and MS rats in an open field paradigm, showing greater anxiolytic behaviour. Altogether, the present study indicates that EE is beneficial in terms of restoring plasma corticosterone levels gradually and reducing anxiety induced by MS stress during SHRP.
Dendritic remodelling in amygdalohippocampal neurons and associated changes in anxiety and ambulatory behaviour in MS rats following EE exposure
Studies across species have further revealed that MS stress during early PND itself could cause changes in the morphology of principal neurons of the hippocampus,[34] anterior cingulate cortex[35], and prefrontal cortex.[36] The dendritic remodelling occurring in these brain regions coincides with effective behavioural changes.[37] The results from the present study observed an increased dendritic arborisation in LA neurons and reduced dendritic arborisation in CA1 neurons following MS stress, indicating that stress effects are not common to all brain regions. Similarly, the changes in dendritic structure are associated with specific changes in behaviour. Following chronic stress, the LA principal neuronal hypertrophy is associated with anxiety,[25,37]; while hippocampal neuronal atrophy was associated with no impact on motor ability but with spatial memory impairments.[38]
Several intervention strategies have been adapted to ameliorate the stress-induced changes in affective behaviour in adult life. One such universal strategy is an exposure to EE exposure with social, sensory and physical stimuli. In the present study, we adapted 10 days of EE at young adulthood to MS rats and showed that how EE was beneficial in ameliorating MS-stress induced changes in the amygdala and hippocampal neurons and associated behaviour. The study has found that the early MS stress effected both anxiety and basal corticosterone in rats with an increased anxiety in an anxiety provoking environment with reduced basal plasma corticosterone. In addition, the study has also found a hypertrophy in LA, while, hypotrophy/atrophy in CA1 hippocampal pyramidal neurons. Treatment with EE has ameliorated the MS stress-induced hypotrophy in dendritic arborisation of CA1 pyramidal neurons. On the other hand, LA pyramidal neurons could not ameliorate MS stress-induced hypertrophy in the apical dendrites completely, but a significant reduction in the dendritic length of basal dendrites of LA pyramidal neurons in both NC and MS rats exposed to EE.
The previous studies showed that both acute and chronic stress in adults cause changes in the dendritic remodelling of the hippocampus and amygdala.[25] However, very few studies are available to discuss how psychological stress including both maternal separation stress and isolation during the critical time of brain development causes changes in the fear circuit and behaviour. The remodelling of dendrites is a common phenomenon in the hippocampus and amygdala following stress. While the hippocampal pyramidal neurons are highly susceptible, but reversible impact to an environmental influence such as acute[39] and chronic stress,[40] hypoxia[41] and ischemic stroke attacks.[42] On the other hand, amygdala is sensitive to stress, but it was found to be irreversible in rats exposed to chronic stress in adulthood.[37] The present study has reinstated the stress effects in the developing brain. The MS stress-induced reduced dendritic arborisation and restoration with 10 days of EE confirms that hippocampal neurons are capable to undergo rigorous dendritic remodelling. The failure to restore the MS stress-induced hypertrophy in LA pyramidal neurons with EE indicating that LA neurons are highly resistant for dendritic remodelling.
Animals that had daily exposure to MS stress during SHRP caused changes in the morphology of dendritic arborisation of both the CA1 hippocampus and LA pyramidal neurons, causing elevated anxiety and repetitive behaviour. CA1 pyramidal neurons in MS rats had less branching and dendritic arborisation than NC pyramidal neurons, whereas LA pyramidal neurons had more branching and dendritic arborisation, which has functional importance. This contrasting pattern in dendritic arborisation between CA1 and LA indicates that the environmental manipulations during SHRP stimulate dendritic growth in LA while shunting the dendritic growth in the CA1 hippocampus. This result is similar to that found in animals subjected to chronic stress during adulthood.[25]
The CA1 pyramidal neurons of NC-EE but not MSEE showed increased apical dendritic branches of CA1 pyramidal neurons at 140–220 µm, whereas LA principal neurons did not have similar increased dendritic branching in apical dendrites. The basal dendrites were unaffected by MS stress, but EE increased the dendritic branching of CA1 pyramidal neurons at 90–200µm, while, reducing the basal dendritic length of LA pyramidal neurons at 40–120µm in both NC-EE and MS-EE rats. In both the CA1 and LA areas, there was no significant difference between NC-EE and MSEE pyramidal neurons.
The EE had restored the dendritic branching pattern in MS rats, suggesting amelioration of dendritic atrophy at the apical dendrites of CA1 pyramidal neurons generated by MS stress at SHRP. The pruning of CA1 pyramidal neurons was more evident at 100–250µm of apical dendrites, while hypertrophy of LA pyramidal neurons was observed mainly at 90–240µm of apical dendrites. The changes in the branching of pyramidal neurons were observed only in the apical but not in the basal dendrites of both LA and CA1 pyramidal neurons.
TAC and LP in MS rats as compared to that of NC
Oxidative stress is one of the major causative factors for stress pathophysiology. Accumulated data suggest the positive association between early-life traumatic events such as maternal separation and long-term oxidative stress alterations eventually contributing to metabolic dysregulation and increased anxiety-like behaviour in animals.[10] In the present study, we investigated the effects of MS stress on biochemical changes in the limbic circuit (amygdala, prefrontal cortex, and hippocampus), adrenal gland, liver, spleen, and blood. The present study sought to compare the reactive oxygen species-induced LP as an indirect measure of oxidative stress for MS stressed animals with age matched control rats. LP is a process of attack by oxidants such as free radicals or non-radical species on lipids with carbon-carbon double bonds (polyunsaturated fatty acids) resulting in lipid peroxyl radicals and hydroperoxides. The accumulation of LP by-products has been implicated in many toxic tissue injuries and pathological processes. Based on its metabolic environment and repair capacities, the cell may opt for its survival or death. Under normal physiological states, the LP rates are at a subtoxic level and cells stimulate their survival through their adaptive stress response supported by its constitutive antioxidant defence systems. During higher levels of peroxidation environment, the repair capacity fails to combat the overwhelming oxidative damage and cells slate themselves toward death.[43,44]
Stress-induced enhancement in LP and the corresponding increase in TAC have been reported earlier.[45] However, there are very few studies indicating the impact of MS stress on oxidative stress. Misiak et al.[46] reported the association between the history of childhood trauma and lipid disturbances and blood pressure in adult first-episode schizophrenia patients. Like higher mammals, in rats, the developing brain is also considered to be more sensitive to stressors of diverse nature. Uysal et al.[47] examined the effects of maternal deprivation on oxidative stress in selected brain tissues (hippocampus, prefrontal cortex, and striatum regions) of infant rats during and after SHRP and found an increased antioxidant enzyme activities and reduced LP during SHRP. Following SHRP, maternal deprivation reduced antioxidant enzyme activities and increased LP, as the possible mechanism through which the infant brain might be protected during SHRP from maternal deprivation-induced oxidative stress. Interestingly, in the present study, we did not find significant changes in LP in PFC, amygdala, and hippocampus, but changes were seen in the adrenal gland of MS rats as compared to NC. On the other hand, we found elevated TAC levels in the liver and spleen, but not in the adrenal gland. As suggested by Diehl et al.[48] to justify their observation on hippocampal oxidative stress, here too an initial enhancement in TAC activity would have helped in this controlled LP.
A positive correlation between TAC and LP was observed in NC groups of amygdala and PFC, which was disturbed when animals were exposed to MS stress due to an increased LP and no change in TAC. In the adrenal gland, a significant TAC-LP correlation in NC (positive) and MS (negative) was observed, with a notably increased LP and decreased TAC in MS. This demonstrates the effects of MS on adrenal gland oxidative stress. No notable variations in TAC/LP in the liver and spleen were observed. Thus, the study indicates that MS stress may not contribute largely to oxidative stress parameters in these organs.
CONCLUSION
Based on the above findings, the present study concludes that the amygdala and hippocampus play an important role in regulating the HPA axis functions and thus on affective behaviour. The augmented dendritic arborisation in the amygdala was resistant to complete restoration; while, blunted hippocampal neurons were able to undergo dendritic remodelling may be responsible for restoring repetitive and ambulatory behaviour.
Acknowledgments
The authors would like to acknowledge Indian Council for Medical Research (ICMR), New Delhi for providing funding support (Ref. No. 55/3/2012/BMS) and NIMHANS for providing the infrastructure facility.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Indian Council for Medical Research (ICMR), New Delhi.
Conflicts of interest
There are no conflicts of interest.
References
- Early-life stress mediated modulation of adult neurogenesis and behavior. Behav Brain Res. 2012;227:400-9.
- [CrossRef] [PubMed] [Google Scholar]
- Extinction of contextual fear with timed exposure to enriched environment: A differential effect. Ann Neurosci. 2017;24:90-104.
- [CrossRef] [PubMed] [Google Scholar]
- Early-life stress alters synaptic plasticity and mTOR signaling: Correlation with anxiety-like and cognition-related behavior. Front Genet. 2020;11:590068.
- [CrossRef] [PubMed] [Google Scholar]
- Early life stress impairs fear conditioning in adult male and female rats. Brain Res. 2006;1087:142-50.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of early maternal separation stress on attention, spatial learning and social interaction behaviour. Exp Brain Res. 2019;237:1993-2010.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of maternal separation and isolation stress during stress hyporesponsive period on fear retention and extinction recall memory from 5-week-to 1-year-old rats. Exp Brain Res. 2019;237:181-90.
- [CrossRef] [PubMed] [Google Scholar]
- A study on fear memory retrieval and REM sleep in maternal separation and isolation stressed rats. Behav Brain Res. 2014;273:144-54.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic social isolation decreases glutamate and glutamine levels and induces oxidative stress in the rat hippocampus. Behav Brain Res. 2015;282:201-8.
- [CrossRef] [PubMed] [Google Scholar]
- Evidences that oxidative stress is linked to anxiety-like behaviour in mice. Brain Behva Immunity. 2008;22:1156-9.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal separation induces long-term oxidative stress alterations and increases anxiety-like behavior of male Balb/cJ mice. Exp Brain Res. 2020;238:2097-107.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic value of a multidisciplinary clinic for intellectual disability. Can J Neurol Sci. 2014;41:333-45.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic stress-induced changes in REM sleep on θ oscillations in the rat hippocampus and amygdala. Brain Res. 2011;1382:155-64.
- [CrossRef] [PubMed] [Google Scholar]
- Stress-induced changes in sleep and associated neuronal activity in rat hippocampus and amygdala. Neuroscience. 2008;153:20-30.
- [CrossRef] [PubMed] [Google Scholar]
- Exercise is brain food: The effects of physical activity on cognitive function. Dev Neurorehabil. 2008;11:236-40.
- [CrossRef] [PubMed] [Google Scholar]
- How the body controls brain temperature: The temperature shielding effect of cerebral blood flow. J Appl Physiol (1985). 2006;101:1481-8.
- [CrossRef] [PubMed] [Google Scholar]
- Long term exposure to combination paradigm of environmental enrichment, physical exercise and diet reverses the spatial memory deficits and restores hippocampal neurogenesis in ventral subicular lesioned rats. Neurobiol Learn Mem. 2016;130:61-70.
- [CrossRef] [PubMed] [Google Scholar]
- Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29:13532-42.
- [CrossRef] [PubMed] [Google Scholar]
- The lighter side of BDNF. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1053-69.
- [CrossRef] [PubMed] [Google Scholar]
- Commentary on the review of measures of early childhood social and emotional development: Conceptualization, critique and recommendtions. J Appl Dev Psychol. 2016;45:19-41.
- [CrossRef] [Google Scholar]
- Trajectories of brain development: Point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3-18.
- [CrossRef] [Google Scholar]
- The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417-63.
- [CrossRef] [Google Scholar]
- Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology. 2007;32:256-66.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434-45.
- [CrossRef] [PubMed] [Google Scholar]
- Generalisation of conditioned fear and its behavioural expression in mice. Behav Brain Res. 2003;145:89-98.
- [CrossRef] [Google Scholar]
- Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810-8.
- [CrossRef] [PubMed] [Google Scholar]
- Determination of absolute hydrogen peroxide concentration by spectrophotometric method. Curr Sci. 2002;83:1193-4.
- [Google Scholar]
- Measurement of the total antioxidant response using a novel automated method in subjects with nonalcoholic steatohepatitis. BMC Gastroenterol. 2005;5:35.
- [CrossRef] [PubMed] [Google Scholar]
- Method for the measurement of antioxidant activity in human fluids. J Clin Pathol. 2001;54:356-61.
- [CrossRef] [PubMed] [Google Scholar]
- Pituitary corticotroph function during the stress hyporesponsive period in neonatal rats. Neuroendocrinology. 1993;57:1003-10.
- [CrossRef] [PubMed] [Google Scholar]
- Stress and the brain: From adaptation to disease. Nat Rev Neurosci. 2005;6:463-75.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of early life stress on anxiety symptoms in late adulthood. Sci Rep. 2019;9:4395.
- [CrossRef] [PubMed] [Google Scholar]
- Acute and chronic effects of exercise on tissue sensitivity to glucocorticoids. J Appl Physiol. 2003;94:869-75.
- [CrossRef] [PubMed] [Google Scholar]
- Compatibility of high-intensity strength and endurance training on hormonal and skeletal muscle adaptations. J Appl Physiol (1985). 1995;78:976-89.
- [CrossRef] [PubMed] [Google Scholar]
- Juvenile emotional experience alters synaptic composition in the rodent cortex, hippocampus, and lateral amygdala. Proc Natl Acad Sci U S A. 2003;100:16137-42.
- [CrossRef] [PubMed] [Google Scholar]
- Juvenile emotional experience alters synaptic inputs on pyramidal neurons in the anterior cingulate cortex. Cereb Cortex. 2001;11:717-27.
- [CrossRef] [PubMed] [Google Scholar]
- Experience-induced changes of dendritic spine densities in the prefrontal and sensory cortex: Correlation with developmental time windows. Cereb Cortex. 2005;15:802-8.
- [CrossRef] [PubMed] [Google Scholar]
- Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667-73.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic stress impairs spatial memory and motivation for reward without disrupting motor ability and motivation to explore. Behav Neurosci. 2006;120:842-51.
- [CrossRef] [PubMed] [Google Scholar]
- Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci U S A. 2008;105:5573-8.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic restraint stress impairs neurogenesis and hippocampus-dependent fear memory in mice: Possible involvement of a brain-specific transcription factor Npas4. J Neurochem. 2010;114:1840-51.
- [CrossRef] [PubMed] [Google Scholar]
- Tualang honey ameliorates hypoxia-induced memory deficits by reducing neuronal damage in the hippocampus of adult male Sprague Dawley rats. Turk J Pharm Sci. 2020;17:555-64.
- [CrossRef] [PubMed] [Google Scholar]
- Transient forebrain ischemia under hyperthermic condition accelerates memory impairment and neuronal death in the gerbil hippocampus by increasing NMDAR1 expression. Mol Med Rep. 2021;23:256.
- [CrossRef] [PubMed] [Google Scholar]
- Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438.
- [CrossRef] [PubMed] [Google Scholar]
- Region-specific effects of maternal separation on oxidative stress accumulation in parvalbumin neurons of male and female rats. Behav Brain Res. 2020;388:112658.
- [CrossRef] [PubMed] [Google Scholar]
- Oxidant/antioxidant effects of chronic exposure to predator odor in prefrontal cortex, amygdala, and hypothalamus. Mol Cell Biochem. 2015;406:121-9.
- [CrossRef] [PubMed] [Google Scholar]
- The history of childhood trauma is associated with lipid disturbances and blood pressure in adult first-episode schizophrenia patients. Gen Hosp Psychiatry. 2015;37:365-7.
- [CrossRef] [PubMed] [Google Scholar]
- Age-dependent effects of maternal deprivation on oxidative stress in infant rat brain. Neurosci Lett. 2005;384:98-101.
- [CrossRef] [PubMed] [Google Scholar]
- Long-lasting effects of maternal separation on an animal model of post-traumatic stress disorder: Effects on memory and hippocampal oxidative stress. Neurochem Res. 2012;37:700-7.
- [CrossRef] [PubMed] [Google Scholar]






