Translate this page into:
A cross-sectional study on clinical correlates of post-stroke depression in a tertiary health-care system of India
*Corresponding author: Srikant Kumar Sahoo, Department of Neurology, IMS and Sum Hospital, SOA Deemed to be University, Bhubaneswar, Odisha, India. drsrikanta25@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Samantray S, Sahoo SK, Mohapatra H. A cross-sectional study on clinical correlates of post-stroke depression in a tertiary health-care system of India. Indian J Physiol Pharmacol 2022;66:62-9.
Abstract
Objectives:
The aim of our study is to determine the relation of the type of stroke and site of lesion and medical comorbidities such as diabetes and hypertension with the severity of depression, HAM-D scores as well as age and gender of these patients.
Materials and Methods:
The present study was a hospital-based cross-sectional study conducted over 2 months that included 61 patients from neurology OPD of IMS and SUM Hospital, Bhubaneswar. Purposive sampling was done. The patients fulfilling the inclusion and exclusion criteria were first assessed using a semi-structured questionnaire to obtain the sociodemographic data. Clinical psychiatric evaluation and detailed mental state examination were done. Based on the clinical findings and using ICD 10-DCR criteria, the 61 patients with CVA were segregated as depressive and non-depressive categories. HAM-A and HAM-D scales were applied on both the groups. MRI findings done at the time of the attack were obtained. Further research is needed to identify the mechanisms of depression and why antidepressants lead to improved physical and cognitive recovery and decreased mortality.
Results:
Age of onset, gender, type of stroke and hemispherical involvement do not show any correlation with PSD. However, we found that the HAM-D scores were much higher in PSD patients with lacunar lesions as compared to non-depressive post stroke patients.
Conclusion:
Lacunar lesions may be involved in the psychopathology of depressive illness among stroke survivors. This could help us predict the occurrence of depressive illness among stroke patients with lacunar lesions. HAM-D and HAM-A may be used to detect anxiety and depressive symptoms among these patients.
Keywords
Post-stroke depression
MRI
Prevalence
Diagnosis
Mortality
INTRODUCTION
In developed countries, stroke is the most common cause for disability, the second leading cause of dementia, and the third leading cause of death. Stroke also predisposes to epilepsy and depression in developed countries. About 20% of survivors require institutional care after 3 months while 15–30% become permanently disabled. Stroke was earlier known as the disease of the developed world. However, low- and middle-income countries constitute 85.5% of total stroke deaths worldwide.[1] Stroke is a clinical syndrome that is divided into two broad types depending on pathophysiology:
Ischaemic strokes occur when there is sudden occlusion of arteries supplying the brain either due to a thrombus formed elsewhere or at the immediate site of occlusion. It is the most common subtype of stroke accounting for almost 85–90% of strokes worldwide.[1] Haemorrhagic strokes are caused due to haemorrhage in the brain. It could be a subarachnoid haemorrhage or intracerebral haemorrhage. This category of stroke accounts for 1–7% and 7–27%, respectively, of all strokes worldwide.[1] A very severe stroke can cause sudden death. The most common symptom of a stroke is a sudden onset of weakness or numbness most often on one side of the body including the face and limbs. Other symptoms consist of confusion, difficulty walking, dizziness, loss of balance or coordination, difficulty seeing with one or both eyes, difficulty speaking or understanding speech, severe headache with no known cause, and fainting or unconsciousness (WHO).
One of meta-analysis of 61 cohorts that included 25,488 patients suggested that the incidence of post-stroke depression (PSD) up to 5 years summed up to 31%.[2] In a previous meta-analysis, it was reported that the cumulative percent of post-stroke survivors who developed depression within the first 5 years was between 39% and 52%.[3] PSD is quite common as it occurs in 33% of stroke survivors during the 1st year after the CVA. Mostly, it is associated with functional impairment or disability and morbid quality of life of the survivors.[4] Stroke severity, site or location of the stroke, and some vascular risk factors have been reported to be associated with PSD. The relative frequency of depression during the acute stage of stroke is more and it decreases with time. Hence, the predictors and severity of depression should be studied separately in acute and chronic stages of stroke.[4]
In one study, the female gender was attributed to have more risk of developing PSD than the male gender. The role of the female gender in PSD is quite controversial. While some studies consider it as an important variable in the prediction the occurrence of depression in stroke survivors,[5,6] however, some other studies suggest that there is no association between female gender and risk of PSD.[3,7,8]
There have been very few studies in the Indian population on determining the clinical correlates and risk factors of PSD. Our study is an attempt to throw some light on how the type and site of stroke affect the occurrence of PSD. It will, in turn, help us to identify the risk factors for developing depression in stroke patients and take necessary measures to prevent it or treat it early in the course. Furthermore, we have tried to analyse the effect of common comorbidities of stroke such as hypertension and Type 2 diabetes mellitus (DM) on PSD.
Aim and objective
The objectives of the study are as follows:
To assess and compare the effect of the site as well as the type of stroke in the brain as well as medical comorbidities such as hypertension and Type 2 DM on the development of the depressive disorder in stroke patients.
To assess and compare the mean Hamilton Depression Rating Scale (HAM-D) scores across age group, gender, site, and type of stroke, and medical comorbidities among PSD patients.
To compare the site as well as the type of stroke, severity of depression, and medical comorbidities between early and late onset stroke patients with comorbid depression.
MATERIALS AND METHODS
The present study was a hospital-based cross-sectional study conducted over 2 months that included 70 patients from neurology OPD of IMS and SUM Hospital, Bhubaneswar. The sample size was in reference to another similar study.[9] However, nine patients did not have MRI scan reports as a result of which data of 61 patients could be obtained. The patients, who were diagnosed with cerebrovascular accidents/stroke by neurologists after clinical evaluation and detailed investigation, were included in the study. Those giving informed and written consent were included in the study. Those with a history of any other debilitating medical or other neurological conditions (except type two DM and hypertension) were excluded as other chronic medical and neurological conditions may also lead to depression and anxiety. Furthermore, patients with pre-existing psychiatric illnesses before stroke were excluded from the study. Patients with comorbid psychotic and manic illnesses were also excluded from the study. The patients who had a recent occurrence of stroke in the past 3 months of the study were excluded so as to avoid depression caused by immediate disability or physical impairment. Sociodemographic data were collected in a semi-structured questionnaire.
Procedure
Purposive sampling was done. The patients fulfilling the inclusion and exclusion criteria were first assessed using a semi-structured questionnaire to obtain the sociodemographic data. A clinical psychiatric evaluation was done with a structured interview and detailed history taking. A detailed mental state examination was done. Based on the clinical findings and using ICD 10-DCR criteria, the 61 patients with CVA were segregated as depressive and non- depressive categories. Psychometric tests such as Hamilton Anxiety Rating Scale (HAM-A) and HAM-D scales were applied on both the groups. MRI findings done at the time of the attack were obtained. The HAM-A is a psychological questionnaire used by clinicians to rate the severity of a patient’s anxiety. The scale consists of 14 items, each defined by a series of symptoms and measures both psychic anxiety (mental agitation and psychological distress) and somatic anxiety (physical complaints related to anxiety). The Hamilton Rating Scale for Depression (HDRS), sometimes also abbreviated as HAM-D, is a multiple-item questionnaire used to provide an indication of depression and as a guide to evaluate recovery. The original version contains 17 items (HDRS17) pertaining to symptoms of depression experienced over the past week. A later 21-item version (HDRS21) included four items intended to subtype the depression.
Statistics analysis
The result was analysed using SPSS 20 and an appropriate statistical test.
RESULTS
The comparison of sociodemographic variables between the two groups (stroke patients with and without depression) using the Chi-square test is done in [Table 1]. There were 27 (62.8%) males and 16 (37.2%) females in stroke patients with depression whereas 12 (66.7%) males and 6 (33.3%) females in stroke patients without depression with a mean age of 62.28 ± 12.60 years in post-stroke depressive group and 64.17 ± 12.43 (SD) years in non-depressive stroke patients group. Thus, the two groups were comparable with respect to age (F = 0.46; P = 0.831) and sex (χ2 = 0.083 and P = 0.774). There were no significant differences statistically with respect to occupational status (χ2 = 1.071 and P = 0.301) and domicile (χ2 = 0.299 and P = 0.585) between the two groups. Among the depressive post-stroke patient group, 46.5% were from rural domicile while 53.5% were from urban domicile. Among the non-depressive group, 38.9% were from rural domicile while 61.1% were from urban domicile. In depressive stroke patients mean score on HAM-D was 20.77 ± 4.61 and on HAM-A, it was 20.51 ± 4.31. In non-depressive stroke patients mean score on HAM-D was 10.39 ± 2.95 and on HAM-A, it was 11.89 ± 3.39. There was a statistically significant difference on HAM-D scores between the two groups with the depressed patients showing a higher mean value as compared to non-depressed stroke patients (F = 7.887, P = 0.007). An Independent t-test has been used for the above comparison.
| Sociodemographic variables | Stroke patients with depression (N=43) n(%) | Stroke patients without depression (N=18) n(%) | χ2 | P |
|---|---|---|---|---|
| Sex | ||||
| Male | 27 (62.8%) | 12 (66.7%) | 0.083 | 0.774 |
| Female | 16 (37.2%) | 6 (33.3%) | ||
| Occupation | ||||
| Employed | 18 (41.9%) | 5 (27.8%) | 1.071 | 0.301 |
| Unemployed | 25 (58.1%) | 13 (72.2%) | ||
| Domicile | ||||
| Rural | 20 (46.5%) | 7 (38.9%) | 0.299 | 0.585 |
| Urban | 23 (53.5%) | 11 (61.1%) | ||
| Mean±SD | Mean±SD | F | ||
| Age (in years) | ||||
| 15–60 years | 62.28±12.60 | 64.17±12.43 | 0.46 | 0.831 |
| HAM-D | 20.77±4.61 | 10.39±2.95 | 7.887** | 0.007 |
| HAM-A | 20.51±4.31 | 11.89±3.39 | 0.394 | 0.533 |
**Significant at 0.01 level. HAM-A: Hamilton Anxiety Rating Scale, HAM-D: Hamilton Depression Rating Scale
[Table 2] displays the comparison of clinical variables between the two groups (depressed and non-depressed stroke patients) using the Chi-square test. There were 15 (34.9%) cases of haemorrhagic stroke and 28 (65.1%) cases of ischaemic stroke among post-stroke depressed patients whereas 5 (27.8%) cases of haemorrhagic stroke and 13 (72.2%) cases of ischaemic stroke among post-stroke non-depressed patients. There was no significant difference statistically with respect to the type of stroke (χ2 = 0.291 and P = 0.590) between the two groups. Among the depressed individuals, 5 (11.6%) cases had bilateral hemispheric involvement, 18 (4.9%) cases where the left hemisphere was affected, and 20 (46.5%) cases with the right hemispherical involvement while in non-depressed stroke patients, bilateral involvement was seen in 3 (16.7%) cases, the left hemisphere was affected in 9 (50%) cases and 6 (33.3%) cases had right hemispherical involvement. There was no significant difference in both the groups regarding the hemispherical involvement (χ2 = 0.953; P = 0.621). Among post-stroke depressed patient, 32 (74.4%) were hypertensive while 11 (25.6%) were non-hypertensive. There were 11 (61.1%) hypertensive and 7 (38.9%) non-hypertensive among non-depressed patients. Among depressed individuals, there were 14 (32.6%) diabetic while 5 (27.8%) were diabetic among non-depressed individuals. Both the groups had no significant difference in medical comorbidities.
| Variables | Stroke patients with depression (N=43) n(%) | Stroke patients without depression (N=18) n(%) | χ2 | df | P |
|---|---|---|---|---|---|
| Stroke type | |||||
| Haemorrhagic | 15 (34.9%) | 5 (27.8%) | 0.291 | 1 | 0.590 |
| Ischaemic | 28 (65.1%) | 13 (72.2%) | |||
| Hemisphere | |||||
| B/L | 5 (11.6%) | 3 (16.7%) | 0.953 | 2 | 0.621 |
| Left | 18 (41.9%) | 9 (50%) | |||
| Right | 20 (46.5%) | 6 (33.3%) | |||
| HTN | |||||
| Yes | 32 (74.4%) | 11 (61.1%) | 1.080 | 1 | 0.299 |
| No | 11 (25.6%) | 7 (38.9%) | |||
| DM | |||||
| Yes | 14 (32.6%) | 5 (27.8%) | 0.135 | 1 | 0.713 |
| No | 29 (67.4%) | 13 (72.2%) |
HTN: Hypertension, DM: Diabetes mellitus
[Table 3] displays the mean HAM-D values across gender, age group, hemisphere, type of stroke, and medical comorbidities among post-stroke depressive patients using an independent t test. There is no significant difference in the mean HAM-D score between male and female gender among PSD patients (P = 0.055). Similarly, there is no difference statistically in mean HAM-D scores between the two age groups (P = 0.589), the two hemispheres (P = 0.165), and between the two types of stroke (P = 0.730). Furthermore, the presence/absence of medical comorbidities such as hypertension (P = 0.831) and Type 2 DM (P = 0.876) do not cause any significant difference in the mean HAM-D scores among PSD patients.
| Post-stroke depression patients (N=43) | HAM-D | P | |
|---|---|---|---|
| Mean | SD | ||
| Sex | |||
| Male | 20.63 | 5.09 | 0.055 |
| Female | 21.00 | 3.80 | |
| Age | |||
| >65 | 19.87 | 4.24 | 0.589 |
| ≤65 | 21.25 | 4.80 | |
| Hemisphere | |||
| Left | 20.00 | 4.10 | 0.165 |
| Right | 21.25 | 5.12 | |
| Type of stroke | |||
| Ischaemic | 20.79 | 4.80 | 0.730 |
| Haemorrhagic | 20.73 | 4.38 | |
| HTN | |||
| Yes | 20.34 | 4.59 | 0.831 |
| No | 22.00 | 4.65 | |
| Type 2 DM | |||
| Yes | 19.71 | 4.80 | 0.876 |
| No | 21.28 | 4.51 | |
HAM-D: Hamilton Depression Rating Scale, HTN: Hypertension, DM: Diabetes mellitus
[Table 4] displays the comparison between early and late onset stroke with respect to hemispherical involvement, type of stroke, the severity on HAM-D, medical comorbidities, and gender among post-stroke depression patients using the Chi-square test. There were 12 (46.67%) cases of haemorrhagic stroke and 16 (53.33%) cases of ischaemic stroke among post-stroke depressed patients of age ≤65 years whereas 13 (46.67%) cases of haemorrhagic stroke and 12 (53.33%) cases of ischaemic stroke among post-stroke depressed patients of age >65 years. There was no significant difference statistically with respect to the type of stroke (χ2 = 2.247 and P = 0.134) between the two groups. Among the PSD patients of age ≤65 years, two cases had bilateral hemispheric involvement, 12 cases where the left hemisphere was affected, and 14 cases with the right hemispherical involvement while in PSD patients of age >65 years, bilateral involvement was seen in three cases, the left hemisphere was affected in six cases and six cases had the right hemispherical involvement. There was no significant difference in both the groups regarding the hemispherical involvement (χ2 = 1.618 and P = 0.445). Among post-stroke depressed patients of age ≤65 years, 22 were hypertensive while six were nonhypertensive. There were ten hypertensive and five nonhypertensive among PSD patients of age > 65 years. Among PSD patients ≤65 years, there were 11 diabetic while three were diabetic among PSD patients >65 years. Both the groups had no significant difference in medical comorbidities (DM: P = 0.198 and HTN: P = 0.394). There were 19 males and nine females among PSD patients ≤65 years while eight males and seven females among PSD patients >65 years. There was no significant difference between the two groups with respect to gender distribution (χ2 = 0.882 and P = 0.348).
| Variables | ≤65 (in years) | >65 (in years) | χ2 | df | P |
|---|---|---|---|---|---|
| Hemisphere | |||||
| Left | 12 (42.9%) | 6 (40%) | 1.618 | 2 | 0.445 |
| Right | 14 (50%) | 6 (40%) | |||
| B/L | 2 (7.1%) | 3 (20%) | |||
| Type of stroke | |||||
| Haemorrhagic | 12 (42.9%) | 13 (52%) | 2.247 | 1 | 0.134 |
| Ischaemic | 16 (57.1%) | 12 (48%) | |||
| Severity on HAM-D | |||||
| Moderate | 8 (28.6%) | 7 | 1.526 | 2 | 0.466 |
| Severe | 9 (32.1%) | 3 | |||
| Very severe | 11 (39.3%) | 5 | |||
| Type 2 DM | |||||
| Yes | 11 | 3 | 1.654 | 1 | 0.198 |
| No | 17 | 12 | |||
| HTN | |||||
| Yes | 22 | 10 | 0.727 | 1 | 0.394 |
| No | 6 | 5 | |||
| Gender | |||||
| Male | 19 | 8 | 0.882 | 1 | 0.348 |
| Female | 9 | 7 |
HAM-D: Hamilton Depression Rating Scale, HTN: Hypertension, DM: Diabetes mellitus
[Table 5] shows the comparison of mean HAM-D scores of various lesion locations between PSD patients and non-depressive stroke patients. There is no significant difference in HAM-D scores between the two groups with respect to the following lesion locations: BASAL GANGLIA, FRONTAL LOBE,[10] HIND BRAIN, and THALAMUS [Figure 1]. However, both the groups show a significant difference in the mean HAM-D scores with respect to lacunar lesions (F = 7.943 and P = 0.030).

- Comparison between post-stroke depression and nondepressive stroke patients across various lesion locations.
| S. No. | Variables | Mean HAM-D score in stroke patients with depression Mean ± SD | Mean HAM-D score in stroke Patients without depression Mean ± SD | F | P |
|---|---|---|---|---|---|
| 1. | BASAL GANGLIA | 19.8 ± 3.4 | 10.6 ± 2.7 | 0.259 | 0.618 |
| 2. | FRONTAL LOBE | 22.8 ± 4.4 | 6.0 ± 5.7 | 0.114 | 0.749 |
| 3. | HIND BRAIN | 20.6 ± 4.5 | 12.0 ± 1.4 | 1.148 | 0.320 |
| 4. | LACUNAR | 24.8 ± 4.0 | 11.7 ± 1.2 | 7.943 | 0.030* |
| 5. | THALAMUS | 17.9 ± 4.9 | 10.0 ± 2.9 | 2.467 | 0.151 |
HAM-D: Hamilton Depression Rating Scale
DISCUSSION
In the present study, the comparison of sociodemographic variables between the two groups (stroke patients with and without depression) using the Chi-square test has been displayed in [Table 1]. The two groups were comparable with respect to age and sex. There is no significant difference in the mean HAM-D score between male and female gender among PSD patients (P = 0.055) as well as between the two age groups that is, ≤65 years and >65 years (P = 0.589). A systemic review consisting of 24 studies reported that gender was not an independent risk factor for PSD similar to our finding. This was seen in 13 out of 21 studies that aimed at finding a correlation between gender and PSD. However, in one-third of these studies female gender was implicated as a risk factor for depression in post-stroke survivors in contrast tour study. There was no association between age in 16 out of 21 studies similar to our study.[11] It was seen in a meta-analysis that the female gender had more risk of developing PSD in acute and sub-acute stages.[12] Few other studies suggested that there was a preponderance of female gender in the occurrence of PSD during the recovery phase which was not seen in our study.[13,14] There were no significant differences statistically with respect to occupational status (χ2 = 1.071 and P = 0.301) and domicile (χ2 = 0.299; P = 0.585) between the two groups. There was a statistically significant difference on HAM-D scores between the two groups with the depressed patients showing a higher mean value as compared to non-depressed stroke patients (F = 7.887 and P = 0.007).
In our study, both the groups had no significant difference in medical comorbidities such as Type 2 DM and hypertension. Furthermore, the presence/absence of hypertension and Type 2 DM do not cause any significant difference in the mean HAM-D scores of PSD patients. In the previous studies, it was found that there was no correlation between major cardiovascular risk factors such as hypertension and hypercholesterolemia with PSD similar to our finding. However, PSD patients were more likely to have DM, unlike our study.[11,15,16] In our study, there was no significant difference with respect to the type of stroke between the PSD group and non-depressed stroke patients. Similarly, there was no significant difference in the mean HAM-D scores between the two types of stroke. Some systematic reviews have not found any correlation between PSD and the type (such as haemorrhagic or ischaemic) which was in accordance to our finding or even the mechanism (such as embolic or thrombotic) of stroke.[11,15,16]
In our study, there was no significant difference in both the groups regarding the hemispherical involvement (χ2 = 0.953 and P = 0.621). Similarly, there is no difference statistically in mean HAM-D scores between the two hemispheres (P = 0.165) among post-stroke depression patients. There is no significant difference in HAM-D scores between the two groups with respect to the following lesion locations: BASAL GANGLIA, FRONTAL LOBE, HIND BRAIN, and THALAMUS [Figures 1 and 2]. However, both the groups show a significant difference in the mean HAM-D scores with respect to lacunar lesions (F = 7.943 and P = 0.030).
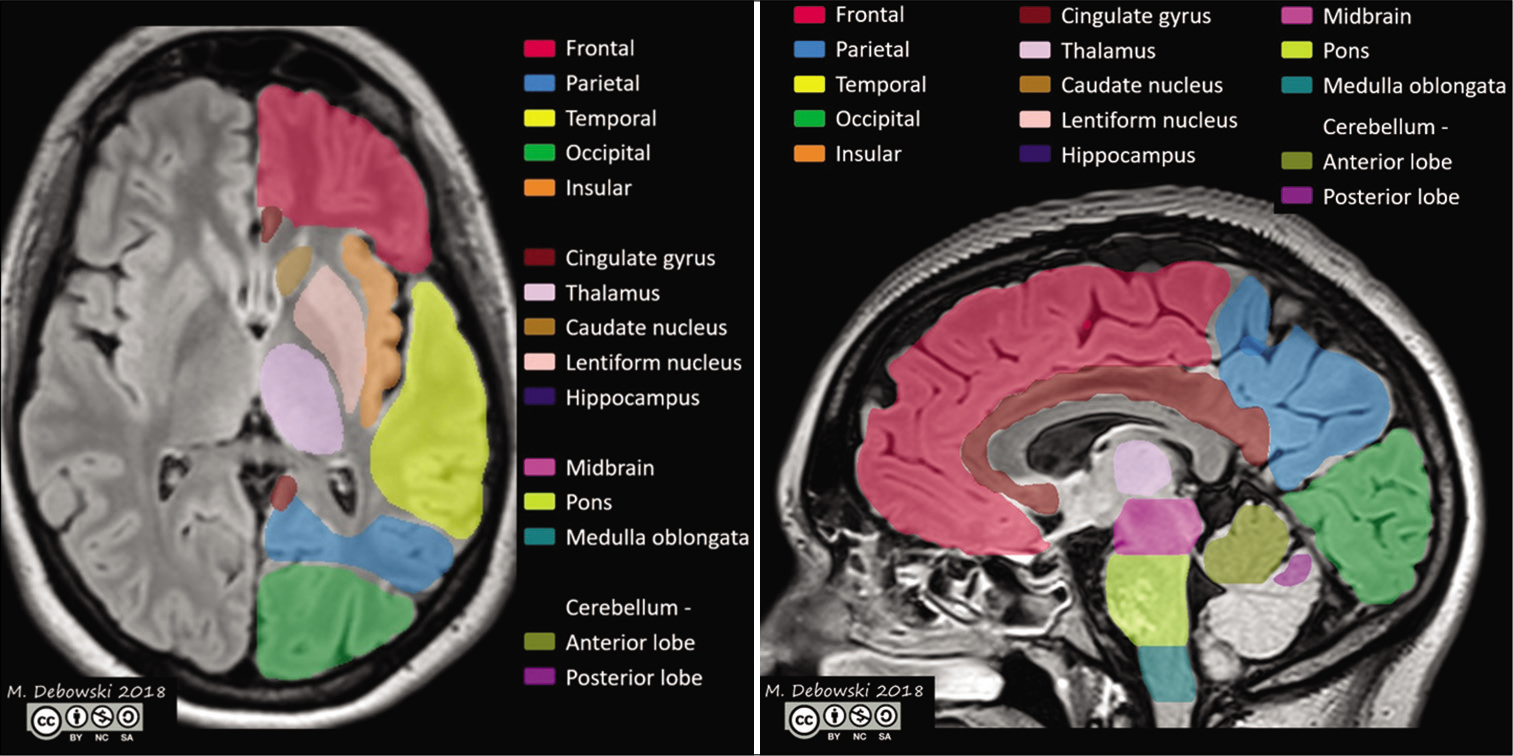
- Lobes and areas of the brain that may be affected in post-stroke depression.
Many attempts have been made to correlate lesion location with the occurrence of PSD;[17] however, the findings have been inconsistent. It was observed that patients with acute stroke who had left basal ganglia or left frontal lesions were more prone to develop minor or major depression as compared to patients with other lesion locations in contrast to our findings.[18,19] These studies also suggested that there was a significant association between the severity of depressive illness and the distance of the ischaemic lesions anterior border from the pole of the left frontal region.[18,19] On the contrary, various meta-analysis thereafter has come to the conclusion that there was no significant correlation of occurrence of depression with lesion location similar to our study.[3,15] Another meta-analysis has also suggested no significant association of the risk of developing depression and the location of the lesion in stroke patients which was in accordance to our finding.[20] Despite conflicting results, some studies have reported that there is a strong association of the left hemispheric lesions of the frontal region that are associated with PSD which is opposite to our results.[21] The hypothesis that PSD is associated with the left basal ganglia or left frontal region of the brain can be supported by the fact that there is evidence of lateralisation of emotions in the brain[22] and the use of rTMS is very effective in vascular depression only when it is administered to the left dorsolateral prefrontal cortex.[23] In another study, it was seen that the left frontal and left BG were associated with the extent of PSD in both acute and sub-acute stages.[12,21,24] This can be explained by the notion that the left hemisphere being the dominant one is responsible for language as well as positive emotions and as the neuroimaging suggests the neurological deficits in the same hemisphere are more severe in the PSD patients.[22] The frontal lobe and BG are responsible for emotional processing; hence, these areas are most likely affected and result in depression.[25] Even though there has been no association between the location of the lesion and PSD.[26-28] some of them[9,19,24,29] have found out that when the lesion is in close contact with that of frontal lobe and left hemisphere, it can lead to PSD.
Physiological perspective
Hasler et al. 2008 have also suggested that in major depressive disorder, there is an abnormal function of the prefrontal cortex and more commonly the left hemisphere of the brain is implicated rather than the right hemisphere.[30] Some newer studies have implicated the limbic-cortical-striatal-pallidalthalamic (LCSPT) circuit in the mechanism of major depressive disorder.[30,31] Drevets et al. (2008) have also claimed that apart from the LCSPT circuit, there are the orbital prefrontal network and medial prefrontal network which are essential for the regulation of emotion and are involved in the occurrence of PSD. The orbital prefrontal network is responsible for various functions such as aversion, relative value, and reward.[31] The medial prefrontal network that is connected to visceral control structures as well as limbic structures helps in various functions such as regulation of emotion, mood, and the reaction of visceral functions to emotion as in neuroendocrine reaction and autonomic regulation.[31] One study has suggested that the prefrontal cortex responds to the treatment of the major depressive disorder.[32] Some studies have utilised MRI findings[9,24] to add to the fact that there is a high prevalence of PSD in lesions affecting the pre-fronto-subcortical circuit mostly of the left hemisphere. Another study found an association between the post-stroke incidence of major depressive episode and lesion volume in the left LCSPT circuit. Another study also revealed that there are five areas of LCSPT such as ventral anterior cingulate cortex, sub-genual cortex, dorsal anterior cingulate cortex, amygdala, and subiculum that were associated with the occurrence of major depressive disorder.[33] The sub-genual cortex, dorsal and ventral anterior cingulate cortex are mostly present near the medial prefrontal cortex suggesting that the frontal lobe can be implicated in the occurrence of PSD.[33] According to one study,[34] lesion in the medial prefrontal cortex (PFC) decreases the ability to shift the focus from one particular work so as to attend to another task and this can explain depressive ruminations in major depressive disorder. Chronic high frequency deep brain stimulation in depressive patients has shown changes in medial PFC activity leading to remission of depressive symptoms.[35] Similarly, CBT has shown changes in medial PFC in depressed patients.[36]
Neuroimaging has suggested that various structural and functional abnormalities are seen in limbic as well as prefrontal regions in major depression.[10,37-39] However, we cannot ignore the presence of depressive illness in post-stroke patients with the involvement of parietal and occipital regions of the brain which are not part of the emotional network. Hence, it suggests that the development of PSD is also dependent on psychological risk factors.[40,41] The disability caused due to stroke leads to enormous stress leading to depressive symptoms.[42,43]
CONCLUSION
Age of onset, gender, type of stroke, and hemispherical involvement do not show any correlation with PSD in our study in contrast to other studies which may be due to certain limitations in this study regarding the sample size. As this is a cross-sectional study, no follow up could be done to assess the course of the disease. However, we found that the HAM-D scores were much higher in PSD patients with lacunar lesions as compared to non-depressive post-stroke patients with similar lesions suggesting that lacunar lesions may be involved in the psychopathology of depressive illness among stroke survivors.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Part I: Frequency of depression after stroke: An updated systematic review and meta-analysis of observational studies. Int J Stroke. 2014;9:1017-25.
- [CrossRef] [PubMed] [Google Scholar]
- Natural history, predictors and outcomes of depression after stroke: Systematic review and meta-analysis. Br J Psychiatry. 2013;202:14-21.
- [CrossRef] [PubMed] [Google Scholar]
- Depression after minor stroke: Prevalence and predictors. J Psychosom Res. 2015;79:143-7.
- [CrossRef] [PubMed] [Google Scholar]
- Depression in acute stroke: Prevalence, dominant symptoms and associated factors. A systematic literature review. Disabil Rehabil. 2011;33:539-56.
- [CrossRef] [PubMed] [Google Scholar]
- The correlates and course of depression in patients with lacunar stroke: Results from the Secondary Prevention of Small Subcortical Strokes (SPS3) study. Cerebrovasc Dis. 2011;32:354-60.
- [CrossRef] [PubMed] [Google Scholar]
- The association between mood and anxiety disorders with vascular diseases and risk factors in a nationally representative sample. J Psychosom Res. 2011;70:145-54.
- [CrossRef] [PubMed] [Google Scholar]
- Depression after minor stroke: Prevalence and predictors. Eur J Neurol. 2012;19:517-21.
- [CrossRef] [PubMed] [Google Scholar]
- Poststroke depression and lesion location revisited. J Neuropsychiatry Clin Neurosci. 2004;16:156-62.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for poststroke depression: Identification of inconsistencies based on a systematic review. J Geriatr Psychiatry Neurol. 2014;27:147-58.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for post-stroke depression: A meta-analysis. Front Aging Neurosci. 2017;9:218.
- [CrossRef] [PubMed] [Google Scholar]
- The Italian multicenter observational study on post-stroke depression (DESTRO) J Neurol. 2006;253:556-62.
- [CrossRef] [PubMed] [Google Scholar]
- Prediction of depressive symptoms up to three years post-stroke. J Rehabil Med. 2009;41:930-5.
- [CrossRef] [PubMed] [Google Scholar]
- Part II: Predictors of depression after stroke and impact of depression on stroke outcome: An updated systematic review of observational studies. Int J Stroke. 2014;9:1026-36.
- [CrossRef] [PubMed] [Google Scholar]
- Poststroke depression incidence and risk factors: An integrative literature review. J Neurosci Nurs. 2006;38:316-27.
- [CrossRef] [PubMed] [Google Scholar]
- Post-stroke depression: A review. Am J Psychiatry. 2016;173:221-31.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of cortical and subcortical lesions in the production of poststroke mood disorders. Brain. 1987;110(Pt 4):1045-59.
- [CrossRef] [PubMed] [Google Scholar]
- Mood disorders in stroke patients. Importance of location of lesion. Brain. 1984;107(Pt 1):81-93.
- [CrossRef] [PubMed] [Google Scholar]
- Post-stroke depression and lesion location: A systematic review. J Neurol. 2015;262:81-90.
- [CrossRef] [PubMed] [Google Scholar]
- Post-stroke depression and lesion location: A hospital based cross-sectional study. Indian J Psychiatry. 2013;55:343-8.
- [CrossRef] [PubMed] [Google Scholar]
- The functional neuroanatomy of emotion and affective style. Trends Cogn Sci. 1999;3:11-21.
- [CrossRef] [Google Scholar]
- Treatment of vascular depression using repetitive transcranial magnetic stimulation. Arch Gen Psychiatry. 2008;65:268-76.
- [CrossRef] [PubMed] [Google Scholar]
- Magnetic resonance imaging correlates of depression after ischemic stroke. Arch Gen Psychiatry. 2001;58:925-31.
- [CrossRef] [PubMed] [Google Scholar]
- Common neural correlates of emotion perception in humans. Hum Brain Mapp. 2015;36:4184-201.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of depression after stroke: A systematic review of observational studies. Stroke. 2005;36:2296-301.
- [CrossRef] [PubMed] [Google Scholar]
- Lesion location and poststroke depression: Systematic review of the methodological limitations in the literature. Stroke. 2004;35:794-802.
- [CrossRef] [PubMed] [Google Scholar]
- Depression after stroke and lesion location: A systematic review. Lancet. 2000;356:122-6.
- [CrossRef] [Google Scholar]
- Major depression in stroke patients. A 3-year longitudinal study. Stroke. 1993;24:976-82.
- [CrossRef] [PubMed] [Google Scholar]
- Neural response to catecholamine depletion in unmedicated subjects with major depressive disorder in remission and healthy subjects. Arch Gen Psychiatry. 2008;65:521-31.
- [CrossRef] [PubMed] [Google Scholar]
- A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628-41.
- [CrossRef] [PubMed] [Google Scholar]
- Anterior cingulate activity as a predictor of degree of treatment response in major depression: Evidence from brain electrical tomography analysis. Am J Psychiatry. 2001;158:405-15.
- [CrossRef] [PubMed] [Google Scholar]
- Stroke lesion in cortical neural circuits and post-stroke incidence of major depressive episode: A 4-month prospective study. World J Biol Psychiatry. 2011;12:539-48.
- [CrossRef] [PubMed] [Google Scholar]
- A cognitive neuroscience hypothesis of mood and depression. Trends Cogn Sci. 2009;13:456-63.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18:1374-83.
- [CrossRef] [PubMed] [Google Scholar]
- Modulation of cortical-limbic pathways in major depression: Treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry. 2004;61:34-41.
- [CrossRef] [PubMed] [Google Scholar]
- Volumetric MRI studies of the hippocampus in major depressive disorder: Meanings of inconsistency and directions for future research. World J Biol Psychiatry. 2010;11:19-35.
- [CrossRef] [PubMed] [Google Scholar]
- Structural brain abnormalities in major depressive disorder: A selective review of recent MRI studies. J Affect Disord. 2009;117:1-17.
- [CrossRef] [PubMed] [Google Scholar]
- Neuroimaging markers of cellular function in major depressive disorder: Implications for therapeutics, personalized medicine, and prevention. Clin Pharmacol Ther. 2012;91:201-14.
- [CrossRef] [PubMed] [Google Scholar]
- Frequency, phenomenology and anatomical-clinical correlates of major post-stroke depression. Br J Psychiatry. 1999;175:163-7.
- [CrossRef] [PubMed] [Google Scholar]
- Relation between depression after stroke, antidepressant therapy, and functional recovery. J Neurol Neurosurg Psychiatry. 2001;71:258-61.
- [CrossRef] [PubMed] [Google Scholar]
- Do family-oriented interventions reduce poststroke depression? A systematic review and recommendations for practice. Top Stroke Rehabil. 2015;22:453-9.
- [CrossRef] [PubMed] [Google Scholar]
- Acute phase factors associated with the course of depression during the first 18 months after first-ever stroke. Disabil Rehabil. 2016;38:30-5.
- [CrossRef] [PubMed] [Google Scholar]






