Translate this page into:
Comparing the quality of life of patients with epilepsy receiving conventional and newer antiepileptics in a tertiary care hospital, Northern India
*Corresponding author: Meenu Thomas, Department of Pharmacology, Teerthanker Mahaveer Medical College and Research Center, Moradabad, Uttar Pradesh, India. mtmuskdeer@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Thomas M, Badyal DK, Pandian JD. Comparing the quality of life of patients with epilepsy receiving conventional and newer antiepileptics in a tertiary care hospital, Northern India. Indian J Physiol Pharmacol. 2024;68:164-71. doi: 10.25259/IJPP_479_2023
Abstract
Objectives:
Both conventional and newer antiepileptic drugs (AEDs) are being used as initial monotherapy in epileptic patients. However, differences in their quality of life (QoL) scores have not been researched considerably. The objective of this study was to determine the overall QoL employing the QoL in Epilepsy Inventory (QOLIE-31) questionnaire
Materials and Methods:
This prospective comparative research was conducted on 126 patients with epilepsy. Two equal groups, A and B, consisting of 63 patients each, were allocated conventional and newer AEDs, respectively. The allotment of the AED was as per the decision of the treating physician based on patient and drug characteristics. QoL was assessed at 0 and 12 weeks using questionnaire QOLIE-31.
Results:
The QoL showed significant improvement with newer AEDs, while conventional AEDs worsened it. The subscales of QOLIE-31 showing the statistical difference in the mean percentage change were worry related to seizure, energy/fatigue, cognitive function, and medication effects. QoL did show a statistically significant difference in young and more educated patients. The patients who had generalised tonic-clonic seizures (GTCS) demonstrated a better QoL than patients with partial seizures, as per the questionnaire.
Conclusion:
The findings of our study suggest that QoL is better with newer AEDs. There seemed to be no effect of gender, age, or education on QoL; however, subjects with GTCS displayed a better QoL. Therefore, newer AEDs can be helpful in improving QoL in epileptic patients. Newer Antiepileptics as monotherapy may offer a better quality of life to epileptic patients.
Keywords
Newer
Conventional
Antiepileptic drugs
Quality of life
Monotherapy
INTRODUCTION
The unforeseeable and recurring seizure episodes distinguish a neurological dysfunction which is called epilepsy.[1] The incidence rate data of epilepsy, as per a systematic review and meta-analysis, suggested an incidence of 61.4/100,000 person-years.[2] Its incidence in our Indian population is reportedly around 1%.[3] The widely used conventional antiepileptic drugs (AEDs) provide extensive familiarity, well-documented adverse drug reactions, and proven efficacy and are usually inexpensive. Nevertheless, it has been observed that around one-fourth of patients receiving AEDs experience treatment failure, and this has triggered enormous research in an attempt to develop newer AEDs.[4] Single-drug therapy is preferred over multi-drug therapy as it is even more efficacious and economical and displays comparatively fewer adverse effects.[5] Since patients with epilepsy often require long-term treatment; hence, their good quality of life (QoL) is crucial.[6]
The QoL in Epilepsy Inventories (QOLIE-31), such as the QOLIE-31, are the frequently used epilepsy-specific tools for measuring QoL in epilepsy.[7] QOLIE-31 has established itself as a benchmark for measuring QoL in people who have epilepsy. It includes 30 question items and one visual analogue scale, which acknowledges seven subcategories, which are worry related to seizures, overall QoL, state of emotional well-being, energy and fatigability, decline in cognition, drug-related effects and social functionality.[8] The promise of a higher QoL is largely influencing the recent trend of adopting newer AEDs.[9] Preceding the laying of the pavement stone of this research, it was observed that there was comparatively not much research comparing QoL with conventional and recently developed AEDs globally as well as in our native population. Hence, the present research intended to comparatively evaluate the QoL of epileptic patients taking conventional and newer AEDs.
MATERIALS AND METHODS
This study was conducted among the outpatients visiting the Neurology Department of Christian Medical College and Hospital, Ludhiana. It was a prospective and open label research in which 126 patients were enrolled after taking informed consent [Annexure 1]. Before patient enrolment, the study received approval from the Institutional Ethics and Research Committee, Christian Medical College and Hospital, Ludhiana. The calculation of the sample size was based on the study by Honari et al.[10] The following formula was used to calculate the sample size for population mean:
Where z = Z statistic for a level of confidence (1.96 for 95% confidence level), α = probability of Type 1 error (0.05), ∂ = population standard deviation, d=precision. Overall QoL was assessed using the QOLIE-31 version 1 questionnaire [Annexure 2] administered before and after 12 weeks. The recruited number of patients remained the same until the end of the study.
Inclusion criteria
The following criteria were included in the study:
Subjects diagnosed as patients with epilepsy according to the international league against epilepsy classification[11]
Either gender patients, aged 18–75 years
Patients willingly giving written informed consent.
Exclusion criteria
The following criteria were excluded from the study:
Subjects suffering from a serious escalating central nervous system disease such as encephalitis or evidence of a demyelinating disorder, severe infection, or an underlying malignancy.[12]
Consequential cardiac conditions such as latest myocardial infarction, severe arrhythmias or congestive heart failure
Any uncontrolled severe illness such as poorly managed diabetes mellitus or any neoplasm
Research drug-related hypersensitiveness
Patients participating in additional research (within 2 months of starting the study or any time while the study is ongoing)
Subjects with evidence of renal or hepatic dysfunction as suggested by serum creatinine of more than 1.5 mg/dL and or level of liver transaminases greater than twice the maximum permitted value
Pregnant ladies and nursing mothers
Subjects having seizures consequent to psychiatric disorders, substance abuse, or delayed mental development.[12]
Patients were divided into two equal groups. Each group consisted of 63 patients. All patients underwent complete general physical examination and basic laboratory evaluation. Sitting blood pressure and pulse rate were assessed. Group A patients were designated to receive the conventional AEDs (sodium valproate, phenytoin and carbamazepine), while Group B patients were allocated the recently developed AEDs (levetiracetam, lamotrigine and oxcarbazepine) as monotherapy. Patients received the appropriate AED as per the decision of the treating physician on the basis of epilepsy type, characteristics unique to the drug as well as the patient. At baseline, the patient’s particulars and details of the disease and drugs were obtained, in addition to the 6-week and 12-week follow-up using the patient’s particular sheet and information from the seizure diary, which was maintained by the patient. QoL was evaluated at 0 weeks and then later 3 months post-therapy using the QOLIE-31 questionnaire.[13]
Statistical analysis
Appropriate statistical tests such as student’s t-test and Chi-square tests were applied. P < 0.05 was considered as statistically significant.
RESULTS
The baseline demographic characteristics at baseline were comparable in both groups A and B, as shown in Table 1. The mean age of subjects in Group A was 35 ± 2 years, and in Group B was 30 ± 2, whereas 27 years was the median age. Concerning profession, a major chunk of the Group A patients (51%) were working, whereas the majority (48%) in Group B were students. In regards to the level of education, the majority of the patients (60% and 79%, respectively) in Groups A and B received education more than 10th standard.
| Characteristics | Group A | Group B |
|---|---|---|
| Total number of patients | 63 | 63 |
| Age in years | 35±2 | 30±2 |
| Sex (M: F) | 37:26 | 30:33 |
| Occupation (%) | ||
| Employed | 51 | 29 |
| Housewives | 22 | 22 |
| Unemployed | 2 | 2 |
| Students | 25 | 48 |
| Education (%) | ||
| <10th standard | 40 | 21 |
| >10th standard | 60 | 79 |
| Smokers | 2 | 2 |
| Alcoholics | 16 | 8 |
Values expressed as percentages and mean±SE, SE: Standard error
The baseline clinical, as well as epilepsy characteristics such as seizure type, average time span of disease (in years), average time span of seizure (in minutes), post-seizure bafflement, status epilepticus and presence of similar history in the household, were similar in both the groups, as shown in Table 2. Table 3 depicts the type of medication received in each group. QoL showed significant improvement in group B, that is, the patients treated with newer AEDs, which were levetiracetam, lamotrigine and oxcarbazepine. The QoL, as per the questionnaire, was enhanced with newer AEDs while, on the contrary, the QoL post-treatment was diminished with conventional AEDs, as depicted in Table 4. Moreover, in the mean percentage change in the scores of subscales, there was statistical significance seen in worry related to seizures and energy/tiredness (both P < 0.05), cognition and medication adverse effects (both P < 0.01). Figure 1 depicts that the absolute values of mean QOLIE-31 scores in Group A at baseline and 12 weeks post-therapy were not statistically significant (P > 0.0 5). However, statistical significance was observed in the absolute values of mean QOLIE-31 scores in Group B at baseline and 12 weeks post-therapy (P < 0.01).
| Characteristics | Group A | Group B |
|---|---|---|
| Clinical characteristics | ||
| Pulse rate (Beat/minute) | 79±8 | 80±6 |
| Weight (Kilograms) | 66±2 | 63±2 |
| Blood pressure (mm of Hg) | ||
| Systolic BP | 114±2 | 113±2 |
| Diastolic BP | 73±1.3 | 73±1.3 |
| Newly diagnosed cases (%) | 68 | 65 |
| Old diagnosed cases (%) | 32 | 35 |
| Epilepsy characteristics | ||
| Generalised tonic-clonic (%) | 65 | 56 |
| Partial (%) | 35 | 37 |
| Unclassified (%) | 0 | 55.5 |
| Mean duration of illness (years) | 6±7 | 6±8 |
| Mean duration of seizure episode (min) | 2.5±0.1 | 2.3±0.1 |
| Post ictal confusion (%) | 73 | 66.7 |
| Status epilepticus (%) | 3 | 8 |
| Positive family history (%) | 14 | 13 |
Values expressed as percentages and mean±SE, BP: Blood pressure, SE: Standard error
| Type of medication | Frequency (n, N=63 each group) |
Percentage |
|---|---|---|
| Group A | ||
| Sodium valproate | 22 | 34.9 |
| Phenytoin | 32 | 50.7 |
| Carbamazepine | 09 | 14.2 |
| Group B | ||
| Levetiracetam | 30 | 47.6 |
| Lamotrigine | 17 | 26.9 |
| Oxcarbazepine | 16 | 25.3 |
| QOLIE-31 scores | Group A | Group B |
|---|---|---|
| Seizure worry | −5.5±4.9 | 13.3±3.2* |
| Overall quality life | 0.01±4.3 | 9.5±3.6 |
| Emotional well-being | −2.6±3.7 | 0.6±3.8 |
| Energy/fatigue | −7.2±4.8 | 6.2±3.3* |
| Cognitive functioning | −34.5±5.9 | 7.4±2.8* |
| Medication effects | −15.4±6.4 | 6.12±5* |
| Social functioning | 5.2±3.5 | 3.1±2.5 |
| Total score | −4.6±2.5 | 1.13±2.4* |
QOLIE: 31 Quality of life in epilepsy questionnaire, *P<0.05 as compared to Group A, values are expressed as mean±SE, $ is mentioned, as (mean percentage change in QOLIE-31) QOLIE-31, SE: Standard error
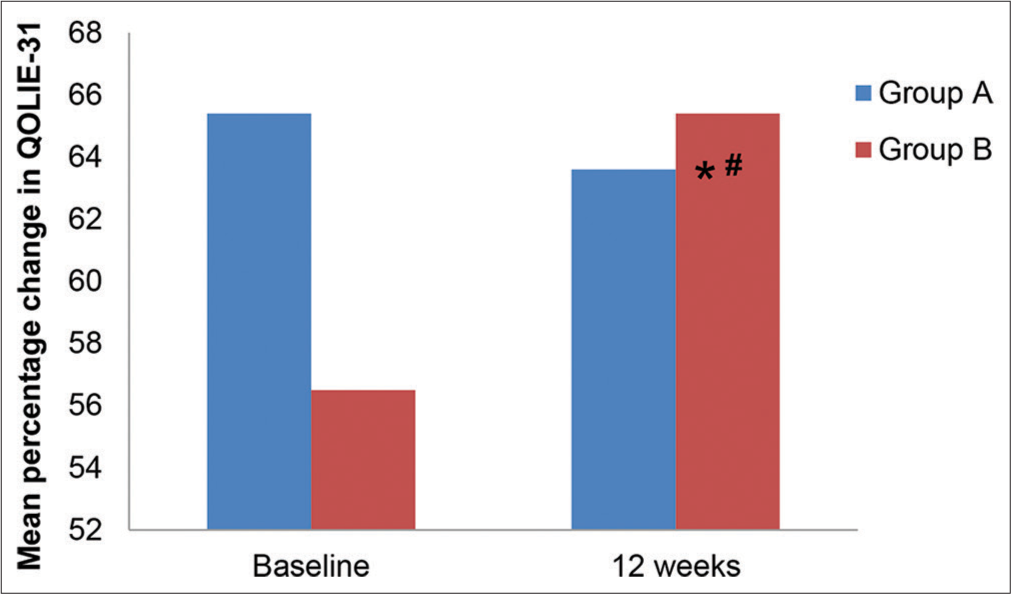
- Total mean quality of life in epilepsy-31 scores at various time intervals in Group A and Group B. QOLIE-31: Quality of life in epilepsy questionnaire. *P < 0.05 as compared to group A, #P < 0.05 as compared to baseline.
Sub-group analysis
Effect of gender on the mean percentage change in QOLIE-31 scores
Gender did not seem to influence the QoL, as suggested by the mean percentage change in QOLIE-31 scores from baseline (P > 0.05), as depicted in Table 5. However, the mean percentage change in QOLIE-31 scores for subscales was significant for worry related to seizure (P < 0.05), cognition (P < 0.001) and medication adverse effects (P < 0.05) for females, while for the males, statistical significance was seen in worry related to seizure (P < 0.05), energy/tiredness (P < 0.05) and cognition (P < 0.001).
| QOLIE-31 scores | Females | Males | ||
|---|---|---|---|---|
| Group A | Group B | Group A | Group B | |
| Seizure worry | −12.9±9.9 | 10.7±4.4* | −0.4±4.6 | 16.2±4.8* |
| Overall quality life | −3.5±8.1 | 6.8±5.4 | 2.5±4.7 | 12.5±4.8 |
| Emotional well-being | −6.6±6.8 | −1.5±4.7 | 0.1±4.1 | 2.9±6.2 |
| Energy/fatigue | −7.2±7.5 | 6.2±4.7 | −6.9±6.4 | 10±4.5* |
| Cognitive functioning | −7.7±7.1 | 2.9±4.3* | −41.1±8.7 | 8.8±3.6* |
| Medication effects | −25.8±12.8 | 2±6.1* | −8.1±6.1 | 10.6±8.1 |
| Social functioning | −6.4±5.0 | 1.78±4 | −4.3±5 | 4.7±3.1 |
| Total score | −7.04±4.1 | 8.57±3.5* | −2.9±3.2 | 1.4±3.1* |
QOLIE: 31 Quality of life in epilepsy questionnaire, *P<0.05 as compared to Group A, Values are expressed as mean±SE, SE: Standard error
Effect of age on mean percentage change in QOLIE-31 scores
Table 6 displays that the QoL did not vary in the two age categories in both the groups, that is, 27 years and below and above 27 years (P > 0.05). However, the mean percentage change in QoL scores for subscales were significant for worry related to seizure (P < 0.05), cognition (P < 0.001) and medication adverse effects (P < 0.05) for patients ≤27 years old while for patients aged more than 27 years statistical significance was depicted in parameters such as worry related to seizure (P < 0.05), energy/tiredness (P < 0.05), cognition (P < 0.001) and social functioning (P < 0.05).
| QOLIE-31 scores | Age <27 years | Age >27 years | ||
|---|---|---|---|---|
| Group A | Group B | Group A | Group B | |
| Seizure worry | −6.4±7.7 | 13.4±5* | −4.8±6.4 | 13.2±3.6* |
| Overall quality life | 4.9±5 | 10.1±5.3 | −4.1±6.8 | 8.7±4.6 |
| Emotional well-being | 0.3±5.1 | 6.2±4.5 | −5.2±5.3 | −7.4±6.5 |
| Energy/fatigue | 1.4±6.3 | 6.6±4.2 | −14.6±7 | 5.7±5.3* |
| Cognitive functioning | −25.2±7.8 | 6.2±4* | −42.5±8.7 | 9.1±3.8* |
| Medication effects | −16.8±10.9 | 6.5±5.4* | −14.2±7.6 | 5.5±9.4 |
| Social functioning | −2.1±5.8 | 2.3±3.8 | −7.3±4.3 | 1±3.1* |
| Total score | −1.4±3.5 | 1.2±3.5* | −7.3±3.6 | 1±2.9* |
QOLIE: 31 Quality of life in epilepsy questionnaire, *P<0.05 as compared to Group A, Values are expressed as mean±SE, SE: Standard error
Effect of education on mean percentage change in QOLIE-31 scores
The QoL in both groups was unaffected by the education level; that is, those educated ≤10th standard and those who received education more than 10th standard had similar QoL, as depicted in Table 7. However, the mean percentage change in QoL scores for subscales was significant for worry related to seizure (P < 0.5), universal QoL (P < 0.05), energy/tiredness (P < 0.05), cognition (P < 0.01) and social functioning (P < 0.05) for patients educated ≤10th standard while for patients educated more than 10th standard statistical significance was seen in seizure-related worry (P < 0.05) and cognition (P < 0.001).
| QOLIE-31 scores | <10th class | >10th class | ||
|---|---|---|---|---|
| Group A | Group B | Group A | Group B | |
| Seizure worry | −1.1±4.9 | 17.8±6.8* | −8.4±7.5 | 12.1±3.7* |
| Overall quality life | −8.1±7.7 | 16.5±5* | 5.3±4.9 | 7.7±4.3 |
| Emotional well-being | −7.3±6.3 | −7.7±7.2 | 0.4±4.5 | 2.7±4.4 |
| Energy/fatigue | −14.8±7.3 | 5.4±6.2* | −2.2±6.3 | 3.8±3.7 |
| Cognitive functioning | −30.8±7.9 | 3.8±7.3* | −37±8.5 | 8.4±3.0* |
| Medication effects | −25.8±11 | −2.6±14.1 | −8.6±7.7 | 8.4±5.1 |
| Social functioning | −11.5±5 | 5.7±3.7* | −1±4.8 | 2.5±3.1 |
| Total score | −7.5±3.9 | 1.3±4.8* | −2.6±3.3 | 1±2.7* |
QOLIE: 31 Quality of life in epilepsy questionnaire, *P<0.05 as compared to Group A, Values are expressed as mean±SE, SE: Standard error
Effect of seizure type on mean percentage change in QOLIE-31 scores
Most of the patients enrolled in both groups had generalised tonic-clonic seizures (GTCS) followed by partial seizures. There was no patient with an unclassified seizure type in Group A. The GTCS patients in both groups had a significantly improved QoL, as shown in Figure 2 and Table 8. The mean percentage change in total QoL scores in patients with GTCS from baseline to 12 weeks in Group A was −3.6 ± 3.1, and in Group B was 1.4 ± 3 (P < 0.001). The mean percentage change in total QOLIE-31 scores in patients with partial seizures from baseline to 12 weeks in Group A was −6.3 ± 4.3, and in Group B was 6.1 ± 4.4 (P = 0.05). The mean percentage change in QOLIE-31 scores for subscales was also significant for worry related to seizure (P < 0.05), cognition (P < 0.001) and medication adverse effects (P < 0.05) for patients with GTCS while for patients with partial seizures, statistical significance was seen only in cognition (P < 0.05).
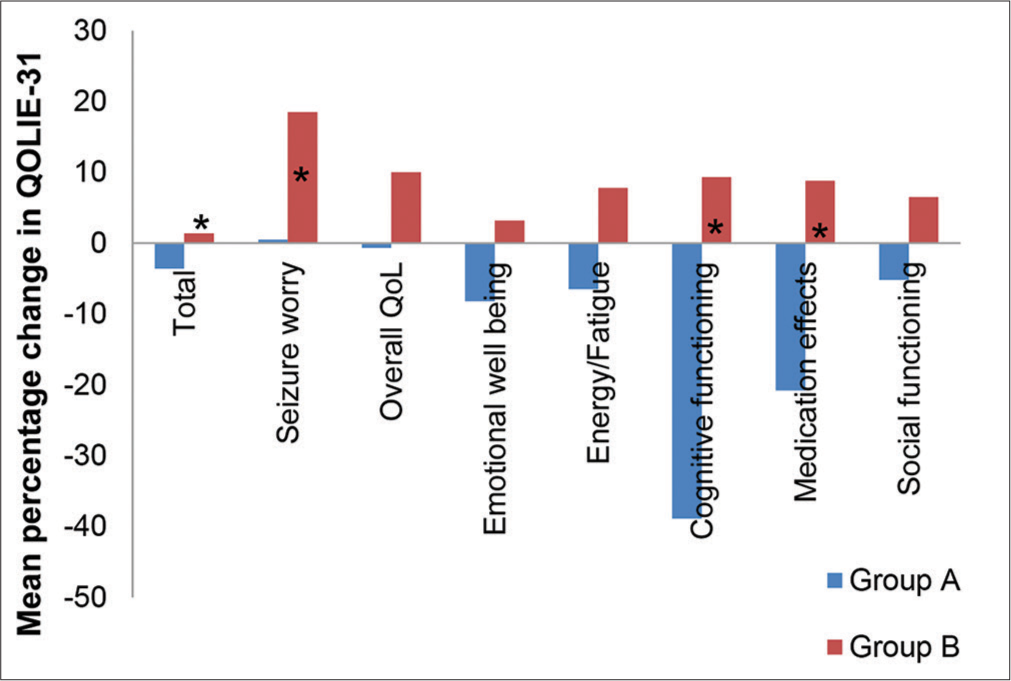
- Mean percentage change in quality of life in epilepsy-31 scores in Group A and Group B in patients with generalised tonic-clonic seizure. QOLIE-31: Quality of life in epilepsy questionnaire, GTCS: Generalised tonic-clonic seizures. *P < 0.05 as compared to group A.
| QOLIE-31 scores | Generalised tonic clonic seizure | Partial seizures | ||
|---|---|---|---|---|
| Group A | Group B | Group A | Group B | |
| Seizure worry | 0.5±5.4 | 18.5±3.9* | −16.9±9.5 | 5±6.3 |
| Overall quality life | −0.7±5.8 | 10±5.4 | 1.4±6.2 | 9.1±5.2 |
| Emotional well-being | −8.2±4.6 | 3.2±4.3 | 7.7±5.6 | −2.7±8.1 |
| Energy/fatigue | −6.5±6.3 | 7.8±4.4 | −8.5±7.5 | 5±5.5 |
| Cognitive functioning | −38.9±7.8 | 9.3±2.9* | −26.3±8.9 | 2.5±5.9* |
| Medication effects | −20.8±9.2 | 8.8±6.8* | −5.4±6.4 | −1.4±8.2 |
| Social functioning | −5.2±4.7 | 6.5±3.3 | −5.2±5.3 | −3.3±4.5 |
| Total score | −3.6±3.1 | 1.4±3* | −6.3±4.3 | 6.1±4.4 |
QOLIE: 31 Quality of life in epilepsy questionnaire, *P<0.05 as compared to Group A, values are expressed as mean±SE, SE: Standard error
DISCUSSION
In addition to facing the great peril of the bodily injuries associated with unforeseeable seizure episodes, an epileptic patient is weighed down by disease-associated social seclusion and stigma.[14] Epileptic patients often display a poorer QoL in comparison to the non-afflicted.[15] The QoL in patients with epilepsy in our research was evaluated by administering the QoL questionnaire, QOLIE-31. This questionnaire incorporates seven scales encompassing the vital concepts of health: emotional health, universal QoL, social functioning, energy/tiredness, cognition, worry related to seizure and medication adverse effects. In addition to the determination of the scores for each scale, the total score was also evaluated per patient.[16]
In our study, a significantly improved QoL with newer AEDs was reflected by the mean percentage change in QOLIE-31 scores from the baseline. Some of the studies support our finding that a newer AED promises a better QoL than a conventional one.[17] Most of the studies have compared absolute values of QoL scores. However, we compared the mean percentage change in QoL scores in both the groups from baseline and found out that newer AEDs, that is, levetiracetam, lamotrigine, and oxcarbazepine had a positive mean percentage change, while the conventional AEDs had a negative mean percentage change in QOLI-31 scores from baseline.
This signifies an improved QoL with newer AEDs in comparison to conventional AEDs, and in fact, it suggests a worsening of the QoL with conventional AEDs. This observation is parallel to finding from another research where the newer AEDs showed a positive change from the baseline in QoL scores from baseline while conventional AEDs showed a negative percentage change in QoL scores from baseline.[18] Although there are some studies suggesting no gross difference in QoL with newer or older drugs, our study suggests otherwise.[19]
The questionnaire subscales utilised in the study have been shown to be highly sensitive in various clinical trials.[20] The subscale which showed the greatest improvement in our study was cognition, and this finding is in alignment with the observation of another research.[21] However, in disagreement with our research findings, another research suggested that the subscale, which improved maximally with newer AED, was medication effects and seizure worry. The effect on cognition of AEDs is very important to be considered since epilepsy and epilepsy syndrome as a disease itself is detrimental to cognitive functions. It can also be interpreted that the results of our study indicate that newer AEDs do not impair cognition as demonstrated by conventional AEDs. Whether they arrest, the cognitive decline or improve cognition is still obscure. Furthermore, the newer AEDs in our study have been shown to improve scores for seizure worry and medication effects, indicating that they alleviate the anxiety and apprehension of recurrence of seizures as well as the fear of injury during a seizure episode or harmful effects of the AEDs.
Our study showed that QOLIE-31 scores did not vary with gender, these findings are similar to another study where the mean total QoL scores did not vary with gender (P > 0.05).[22] Our results differ from another study where the female gender was found to be a key influence on improved QoL.[23] However, another study suggested significantly lower QOLIE-31 scores in females than in males in dimensions such as worry related to seizure, emotional health, energy/tiredness, cognition and overall scores.[24] One study showed decreased QoL in male epileptics as compared to females.[25] No difference in QoL with gender suggests an improved scenario in our country where the position of females in society seems to be refined, updated and well-acknowledged.
The mean percentage change in QOLIE-31 scores did not vary significantly with age this is in accordance with another study where age did not impact the QoL scores significantly.[26] However, another study showed higher QoL scores in younger epileptics.[27] Some studies showed that elderly patients possess better coping capabilities and, therefore, display a better QoL in certain areas than young epileptics.[28] The mean percentage change in QOLIE-31 scores did not vary significantly with education in our study, whereas, in another study, better QoL was associated with more advanced education.[29] Our research did not reflect a significantly higher QoL with higher education, which should have been there, expecting that the more educated patients would be more aware and well versed with the disease as well as the AEDs used and also would have less stigma associated with the disease. However, the increasing dependence and over and mis-utilisation of social media and platforms like Google for seeking medical information often creates an unwanted and out-of-proportion health scare and can adversely affect QoL, too.
Our study reflected a significant improvement in QoL scores in patients with GTCS but not in partial seizures. Our research observations are parallel to those from another research, which found that patients with partial seizures had poorer QoL than patients with GTCS.[30] However, in another study, the QoL scores did not vary significantly with the type of epilepsy.[31] Although one would anticipate lower QoL scores in patients with GTCS due to associated with loss of consciousness and vulnerability to seizure related injury, however, in contrast, in an episode of a partial seizure, the patient remaining conscious throughout the ordeal makes them more apprehensive concerning the next episode and consequently adversely affecting QoL.
Our study is not without limitations. One limitation is the small sample size; however, this study, being one of the preliminary studies to gather the data of AED as a group rather than a head-on comparison of a single AED, offers relatively new information. In addition, the inclusion of alcoholics and smokers in our study could have an impact on the results as these are well documented to cause interactions with AEDs.[32] There are limited studies comparing QoL with older and latest antiepileptics worldwide. In the Indian population, the database for such comparative studies did not yield much data. Even so, most of the few such studies conducted made a comparison of a few drugs only and not as a group. This study was a preliminary step to build up threshold research evidence on which further research can be built.
CONCLUSION
Our research observed the newer AEDs to be beneficial in improving QoL in epileptic patients.
Acknowledgment
‘Gratitude is the fairest blossom which springs from the soul.’
Henry Ward Beecher
I want to acknowledge and place on record my sincere thanks to all those who were instrumental directly or indirectly in shaping my academic career and in completing this research work successfully. With joy and gratitude, I offer thanks and praise to God almighty for my very existence as well as his showers of blessings on me and for bestowing his grace and strength toward the successful completion of this work. I cannot express in words the debt I owe to my teacher and mentor, Dr. Dinesh K Badyal, M.D., for his keen interest, encouragement and guidance. Without his supervision at every step, this work would not have been successfully completed. I take this opportunity to especially express my thanks to Dr. Jeyaraj D. Pandian, M.D., D.M., Department of Neurology, for his unconditional help, guidance and support in this research work. His suggestions were of tremendous help to me in analysing my observations of the study.
I wish to thank my family for their constant support and encouragement throughout the research process. It is not possible to acknowledge all the subjects in the study who willingly gave their consent and allowed me to perform this work. Without them, this work would not have been possible. Finally, I register my thanks to all my patients and those who helped me and supported me in every possible way during the progress of my research work.
(Dr. Meenu Thomas)
Ethical approval
The research/study approved by the Institutional Review Board at Institutional Ethics and Research Committee, Christian Medical College and Hospital, Ludhiana, number CMC/3525, dated 10 April 2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Supplementary data available at:
Financial support and sponsorship
Nil.
References
- The epidemiology of epilepsy. Neuroepidemiology. 2020;54:185-91.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and incidence of epilepsy: A systematic review and meta-analysis of international studies. Neurology. 2017;88:296-303.
- [CrossRef] [PubMed] [Google Scholar]
- Epilepsy: Indian perspective. Ann Indian Acad Neurol. 2014;17:S3-11.
- [CrossRef] [PubMed] [Google Scholar]
- Nuevos fármacos antiepilépticos [New antiepileptic drugs] Medicina (B Aires). 2019;79(Suppl 3):48-53.
- [Google Scholar]
- Determinants of quality of life in adults living with epilepsy. Ann Afr Med. 2020;19:164-9.
- [CrossRef] [PubMed] [Google Scholar]
- Translation and cross-cultural adaptation of the 'Quality of Life in Epilepsy (QOLIE-31-P)' questionnaire for Chile. Epilepsy Behav. 2021;122:108169.
- [CrossRef] [PubMed] [Google Scholar]
- Determinants of quality of life in adults with epilepsy: A multicenter, cross-sectional study from Germany. Neurol Res Pract. 2023;5:41.
- [CrossRef] [PubMed] [Google Scholar]
- Quality of life for patients with epilepsy is determined by more than seizure control: The role of psychosocial factors. Expert Rev Neurother. 2006;6:111-8.
- [CrossRef] [PubMed] [Google Scholar]
- Epilepsy and quality of life in Iranian epileptic patients. J Patient Rep Outcomes. 2021;5:16.
- [CrossRef] [PubMed] [Google Scholar]
- Seizures and epilepsy: An overview for neuroscientists. Cold Spring Harb Perspect Med. 2015;5:a022426.
- [CrossRef] [PubMed] [Google Scholar]
- Antiepileptic drugs and quality of life in patients with epilepsy: A tertiary care hospital-based study. Value Health Reg Issues. 2015;6:1-6.
- [CrossRef] [PubMed] [Google Scholar]
- Health-related quality of life in double-blind Phase III studies of brivaracetam as adjunctive therapy of focal seizures: A pooled, post-hoc analysis. Epilepsy Behav. 2017;69:80-5.
- [CrossRef] [PubMed] [Google Scholar]
- Correlates of perceived stigma for people living with epilepsy: A meta-analysis. Epilepsy Behav. 2017;70:198-203.
- [CrossRef] [PubMed] [Google Scholar]
- Perceived quality of life (QOLIE-31-P), depression (NDDI-E), anxiety (GAD-7), and insomnia in patients with epilepsy attended at a refractory epilepsy unit in real-life clinical practice. Neurol Sci. 2022;43:1955-64.
- [CrossRef] [PubMed] [Google Scholar]
- The quality of life of people with epilepsy at a tertiary referral centre in Malaysia. Health Qual Life Outcomes. 2013;11:143.
- [CrossRef] [PubMed] [Google Scholar]
- Antiepileptic drugs in development pipeline: A recent update. eNeurologicalSci. 2016;4:42-51.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of levetiracetam and carbamazepine as monotherapy in partial seizures. Epilepsy Res Treat. 2015;2015:415082.
- [CrossRef] [PubMed] [Google Scholar]
- Quality of life in adult patients with epilepsy and their family members. Seizure. 2013;22:128-35.
- [CrossRef] [PubMed] [Google Scholar]
- Lamotrigine monotherapy compared with carbamazepine, phenytoin, or valproate monotherapy in patients with epilepsy. Epilepsy Behav. 2003;4:659-66.
- [CrossRef] [PubMed] [Google Scholar]
- Neurocognitive effects of antiseizure medications in children and adolescents with epilepsy. Paediatr Drugs. 2021;23:253-86.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical profile of psychogenic non-epileptic seizures in adults: A study of 63 cases. Ann Indian Acad Neurol. 2013;16:157-62.
- [CrossRef] [PubMed] [Google Scholar]
- Calidad de vida en pacientes adultos con epilepsia generalizada idiopática. Estudio EPILAK [Quality of life in adult patients with idiopathic generalised epilepsy. The EPILAK study] Rev Neurol. 2021;72:195-202.
- [CrossRef] [PubMed] [Google Scholar]
- Epilepsy and quality of life: Socio-demographic and clinical aspects, and psychiatric co-morbidity. Arq Neuropsiquiatr. 2013;71:385-91.
- [CrossRef] [PubMed] [Google Scholar]
- Health-related quality of life in adolescents with epilepsy in Montenegro. Epilepsy Behav. 2017;76:105-9.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of quality of life and associated factors among people with epilepsy attending at Amanuel mental specialized hospital, Addis Ababa, Ethiopia. Sci J Public Health. 2014;2:378-83.
- [CrossRef] [Google Scholar]
- Health-related quality of life in adults with epilepsy: The effect of age, age at onset and duration of epilepsy in a multicentre Italian study. BMC Neurol. 2011;11:33-45.
- [CrossRef] [PubMed] [Google Scholar]
- Factors associated with impaired quality of life in younger and older adults with epilepsy. Epilepsy Res. 2009;83:58-65.
- [CrossRef] [PubMed] [Google Scholar]
- Quality of life and its associated factors among epileptic patients attending public hospitals in North Wollo Zone, Northeast Ethiopia: A cross-sectional study. PLoS One. 2021;16:e0247336.
- [CrossRef] [PubMed] [Google Scholar]
- Quality of life in patients with epilepsy in India. J Neurosci Rural Pract. 2011;2:33-8.
- [CrossRef] [PubMed] [Google Scholar]
- Depression and quality of life in patients with epilepsy-single centre experience. Psychiatr Danub. 2021;33:486-9.
- [Google Scholar]
- How do smoking, vaping, and nicotine affect people with epilepsy and seizures? A scoping review protocol. PLoS One. 2023;18:e0288120.
- [CrossRef] [PubMed] [Google Scholar]






