Translate this page into:
Increased angiotensin converting enzyme (ACE2) level is associated with the cardiovascular risk in diabetes
*Corresponding author: Meera Shivasekar, Department of Biochemistry, SRM Medical College Hospital and Research Center, Chennai, Tamil Nadu, India. meeras@srmist.edu.in
-
Received: ,
Accepted: ,
How to cite this article: Naveena G, Shivasekar M, Vinodhini VM, Nath D. Increased angiotensin converting enzyme 2 level as an early marker of cardiovascular risk in diabetes. Indian J Physiol Pharmacol. 2025;69:77-82. doi: 10.25259/IJPP_288_2024
Abstract
Objectives:
Increased plasma angiotensin-converting enzyme 2 (ACE2) levels are associated with cardiovascular risk in diabetes and therapeutic targets. ACE2 is an endogenous regulator of the renin-angiotensin system and is important in various cardiovascular diseases (CVDs). This study aimed to investigate the association between increased ACE2 levels and cardiovascular risk in patients with type 2 diabetes mellitus (T2DM), particularly those with CVD.
Materials and Methods:
In this cross-sectional study, subjects were classified into 3 groups. Each Group has 87 individuals. Group I was considered as controls, Group II was considered as diabetes, and Group III was considered as diabetes with heart disease who attended the master health checkup, cardiology and general medicine OP in the tertiary care centre. The serum ACE, ACE2, oxidized-low-density lipoprotein (ox-LDL), and highly sensitive c-reactive were examined using the enzyme-linked immunosorbent assay technique, and the lipid levels were measured using the AU 480 (Auto analyser).
Results:
The study found that mean plasma ACE, ACE2, ACE/ACE2 ratio, ox-LDL, and high-sensitivity-C-reactive protein (hs-CRP) levels were significantly higher in group III than compared to group II and group I (P < 0.001). A significant correlation was observed between ACE2, ACE, ACE/ACE2 ratio, hs-CRP, and ox-LDL in patients with group III. Linear regression analysis showed a strong association of ACE2 with hs-CRP, ox-LDL and ACE.
Conclusion:
The study concludes that increased ACE2 levels may be associated with the risk of CVD in T2DM, especially in those with coronary heart disease. These findings suggest the possibility of independent risk assessment in diabetic patients.
Keywords
Angiotensin converting enzyme 2
Coronary heart disease
Renin angiotensin system
Type 2 diabetes mellitus
INTRODUCTION
Coronary heart disease (CHD) is a major comorbidity in patients with type 2 diabetes mellitus (T2DM), leading to elevated morbidity and mortality.[1] The renin-angiotensin-aldosterone system plays a crucial role in cardiovascular regulation. Angiotensin-converting enzyme 2 (ACE2), a type I transmembrane glycoprotein with 805 amino acids, is 42% similar to that of ACE. ACE2, a homologue of ACE, is a monocarboxypeptidase that preferentially removes carboxy-terminal amino acids from various substrates, including angiotensin I (Ang I) and angiotensin II (Ang II).[2] ACE converts angiotensin I to angiotensin II is a vasoconstrictor that induces oxidative stress and hypertension. On the other hand, ACE activity is counterbalanced by ACE2, which is converted into Ang II to Ang-(1–7) and is a vasodilator. ACE/Angiotensin II (AngII) and ACE2/angiotensin 1–7 (Ang1–7) are two functional axes that work together to protect body homeostasis by preserving a dynamic equilibrium.[3] ACE2 is released into circulation via cleavage by tumour necrosis factor-alpha converting enzyme (TACE), resulting in increased tissue levels of Ang II and decreased Ang-(1–7) levels. ACE2 is located in the endothelium of microcirculation and is present in atherosclerotic radial arteries.[4] ACE2/Ang1–7 exerts a protective function in the dedifferentiation of beta cells by enhancing the microcirculation of islets and suppressing the involvement of islet inducible nitric oxide synthase.[5] The imbalance in the ACE/ACE2 ratio has been implicated in various pathological conditions, correlating with worse clinical outcomes. Specifically, ACE2 contributes to an elevated ACE/ACE2 ratio, which has been observed in cardiovascular diseases (CVDs).[6] In healthy subjects, circulating ACE2 activity levels are low but increase in the presence of CVDs such as hypertension and heart failure. Under pathological circumstances, ACE/Ang II can cause enhanced insulin resistance in diabetes mellitus (DM), inflammation, vasoconstriction, and oxidative stress.[7] Therefore, ACE2/Ang1–7 and ACE/AngII have a state of dynamic balance disorder developed ACE/ACE2 ratio dysfunction in diabetes, which may be related to the influence of high blood glucose on the systemic inflammatory response and immune system dysfunction.[8] The expression of ACE and ACE2 significantly affects many pathophysiological effects of the renin-angiotensin system (RAS) and has a relationship with CHD.[9] The study aimed to investigate the utility of plasma ACE2 activity levels to predict cardiovascular events in T2DM.
MATERIALS AND METHODS
Study subjects
This cross-sectional study was conducted in patients attending the Medicine, Cardiology and Master Health Checkup outpatient (OP) at SRMMCH and RI Tamil Nadu, India. Based on the prevalence rate, the study was categorised into three groups. Group-I Controls (n = 87), Group-II DM (n = 87) and Group-III Diabetics with CHD (n = 87). The study included T2DM patients based on American Diabetes Association criteria fasting plasma blood glucose (FPBG) levels of ≥126 mg/dL, and the Hemoglobin A1c (HbA1c) of ≥6.5% and also based on Electrocardiogram, ST-levels elevation, and abnormal coronary angiography findings with more than 50% stenosis in one or more major arteries, and were involved in this study. Patients with chronic conditions, such as liver failure, cancer, pregnancy, heart failure, cardiomyopathy, and cardiovascular accidents, were excluded.
Ethical consideration
The study investigation was approved by the institutional ethical (IEC No. 8717/IEC/2023). Every study participant gave their informed consent.
Ascertainment of covariates
The study observed higher circulating levels of ACE2 in patients with T2DM and T2DM with CHD. Anthropometric variables, including weight and height, were measured using standard equipment and methods. After a period of rest, at intervals of one minute, the resting blood pressure (BP) was taken in three sitting positions: Hypertension with systolic BP (SBP) ≥140 mmHg and diastolic BP ≥90 mmHg T2DM before medical diagnosis based on FPBG levels of ≥126 mg/dL, and the HbA1c of ≥6.5%. The samples were taken after a minimum 12-h fast and an overnight period of rest without exertion. A venous blood sample was collected from the participants. Blood samples were centrifuged at 3000 rpm and refrigerated at −20° C until analysis. Fasting glucose and serum lipid profile levels were measured enzymatically using an AU480 automatic analyser (Beckman Coulter, Brea, CA, USA). Sandwich Enzyme-linked immunosorbent assay (ELISA) was used to determine the concentrations of ACE, ox-low-density lipoprotein (ox-LDL), high-sensitivity C-reactive protein (hs-CRP), and ACE2 measured by calorimetrically.
ACE and ACE2 assay
The ACE was measured using sandwich enzyme-linked sorbent assay (Krishgen biosystem catalogue no: KBH0927), and ACE2 was determined using the ELISA method (Krishgen biosystem catalogue no: KBH3169).
hs-CRP assay
The serum hs-CRP was measured by the sandwich ELISA BioSource Laboratory (Catalogue No: MBS2506093).
Ox-LDL assay
Serum ox-LDL was measured according to manufacturing protocol. The concentration of ox-LDL was determined by a sandwich enzyme-linked sorbent assay (E1542Hu, Abbkine Scientific Co., Ltd., Wuhan, China).
Statistical analysis
The Statistical Package for the Social Sciences version 22 (IBM Corp’s Armonk, NY, USA) was used for the statistical analysis. The mean and standard deviation are used to express quantitative variables. Descriptive analysis was done using one-way analysis of variance (ANOVA) to compare mean values for continuous variables. Pearson’s correlation coefficient (r) was used to assess the relationships between variables (ACE, ACE2, ox-LDL, fasting blood glucose [FBG], insulin, HbA1c and lipid profiles). Linear regression analysis was carried out using ACE2 as a dependent variable and taking independent variables to assess the contribution of ACE2 to the prediction of T2DM of each parameter. P < 0.005 is statistically significant.
RESULTS
This study included 261 participants and was divided into 3 groups. Group-I (control), Group-II (T2DM) and Group-III (T2DM + CHD). There were 60 males and 27 females in group-III subjects. As group-II subjects, 59 were males, and 28 were females, and as group-I subjects, 45 were males, and 42 were females. The majority are between the age group of 35–60 years. Table 1 shows the demographic and quantitative data of all three groups. Statistically significant differences were found in participants’ age, body mass index, waist circumference, SBP, W/H ratio, FBG, and HbA1c in Group III and Group II when compared with Group I. Table 2 shows the biochemical and lipid profile. A significant difference in total cholesterol (TC), LDL, high-density lipoprotein (HDL), very LDL, TC/HDL ratio, LDL/HDL ratio, hs-CRP, ACE, ACE2, and oxidised LDL (P < 0.001).
| Parameter | Control group 1 (n=87) | Diabetes mellitus group 2 (n=87) | Diabetic with CHD (DM+CHD) Group 3 (n=87) | P-value |
|---|---|---|---|---|
| Age (years) | 46.2±8.01 | 44.2±8.01 | 46.1±9.5 | 0.014 |
| Gender M/F | 45/42 | 59/28 | 60/27 | − |
| Height (cm) | 170.7±3.6 | 170.83±3.8 | 171.5±4.09 | 0.338 |
| Weight (kg) | 70.83±7.56 | 73.1±7.8 | 75.1±7.81 | 0.002 |
| BMI (kg/m2) | 23.49±5.4 | 24.63±5.7 | 24.75±4.07 | 0.005 |
| WC (cm) | 86.14±5.2 | 89.62±5.4 | 90.52±4.05 | <0.001 |
| HC (cm) | 99.63±3.05 | 101.1±4.2 | 100.5±4.6 | 0.053 |
| W/H ratio | 0.94±0.035 | 0.89±0.042 | 0.91±0.04 | <0.001 |
| Systolic BP (mmHg) | 117.95±4.2 | 124.27±3.24 | 129.18±3.7 | <0.001 |
| Diastolic BP (mmHg) | 80.97±1.87 | 79.20±7.21 | 79.20±6.92 | 0.074 |
| Duration of disease (years) | 0 | 3.271±1.26 | 5.61±2.26 | <0.001 |
P < 0.05 is statistically significant. One-way analysis of variance calculation. CHD: Coronary heart disease, DM: Diabetes mellitus, BP: Blood pressure, BMI: Body mass index, WC: waist circumference, HC: Hip circumference, W/H: Waist/hip ratio
| Parameter | Control Group 1 (n=87) | Diabetes Mellitus Group 2 (n=87) | Diabetic with CHD (DM+CHD) Group 3 (n=87) | P-value |
|---|---|---|---|---|
| FBG (mg/dL) | 98.88±7.11 | 198.55±9.02 | 217.74±64.75 | <0.001 |
| HbA1c (%) | 4.94±0.89 | 7.19±1.68 | 9.56±3.30 | <0.001 |
| TC (mg/dL) | 143.48±12.36 | 221.87±18.13 | 230.79±20.58 | <0.001 |
| TGL (mg/dL) | 107.26±8.64 | 177.68±10.12 | 210.48±9.37 | <0.001 |
| LDL-C (mg/dL) | 98.83±7.86 | 159.83±18.86 | 163.38±20.19 | <0.001 |
| VLDL-C (mg/dL) | 27.25±7.72 | 32.27±15.97 | 37.90±15.23 | <0.001 |
| HDL-C (mg/dL) | 45.40±3.076 | 38.68±2.31 | 36.29±1.71 | <0.001 |
| TC/HDL | 3.56±1.005 | 5.65±1.37 | 6.85±1.10 | <0.001 |
| LDL/HDL | 2.89±0.83 | 4.13±0.80 | 4.55±0.71 | <0.001 |
| ACE/ACE2 ratio | 0.47±0.02 | 1.29±0.15 | 2.25±0.29 | <0.001 |
| hs-CRP (mg/L) | 0.585±0.79 | 3.91±1.41 | 5.22±1.26 | <0.001 |
| Ox-LDL (ug/L) | 23.66±3.36 | 38.27±8.38 | 42.28±2.07 | <0.001 |
| ACE (pg/mL) | 42.92±2.04 | 78.56±9.56 | 97.67±10.33 | <0.001 |
| ACE2 (pg/mL) | 30.73±5.79 | 70.82±12.17 | 80.90±11.28 | <0.001 |
P-value <0.05 is statistically significant. One-way analysis of variance calculation. FBG: Fasting blood glucose, HbA1c: Hemoglobin A1c, TC: Total cholesterol, TGL: Triglycerides, LDL-C: Low-density lipoprotein cholesterol, VLDL-C: very low density lipoprotein cholesterol, HDL-C: high-density lipoprotein cholesterol, ACE2: Angiotensin Converting Enzyme 2, hs-CRP: high-sensitivity C-reactive protein, Ox-LDL: Oxidized-low-density lipoprotein
Table 3 shows Pearson’s correlation between plasma ACE, ACE2, FBG, ACE/ACE2 ratio, hs-CRP, and ox-LDL in group III. A positive correlation between ACE2 and FBG (r = 0.4992, P < 0.0001), and ox-LDL (r = 0.5784, P < 0.0001) and, hs-CRP (r = 0.4583, P < 0.0001) and ACE (r = 0.4615, P < 0.0001) and ACE/ACE2 ratio (r = 0.2173, P = 0.0432).
| Parameters | ACE2 | |
|---|---|---|
| r- value | P-value | |
| FBG (mg/dl) | 0.4992 | <0.0001 |
| Ox-LDL (ug/L) | 0.5784 | <0.0001 |
| ACE/ACE ratio | 0.2173 | 0.0432 |
| hs-CRP (mg/L) | 0.4583 | <0.0001 |
| ACE (U/L) | 0.4615 | 0.0002 |
A P < 0.05 is significant. T2DM: Type 2 Diabetes mellitus, CHD: Coronary heart disease, Ox-LDL: Oxidized-low-density lipoprotein, ACE: Angiotensin converting enzyme, hs-CRP: high-sensitivity C-reactive protein, FBG: Fasting blood glucose
The linear regression analysis of ACE2 with hs-CRP (r = 0.458), ACE (r = 0.1479) and ox-LDL (r = 0.8409) in group-III individuals is shown in Figure 1. A significant positive correlation was observed between hs-CRP and oxLDL with ACE2 in group- III. Group- III subjects exhibit a strong significant association with ACE2.
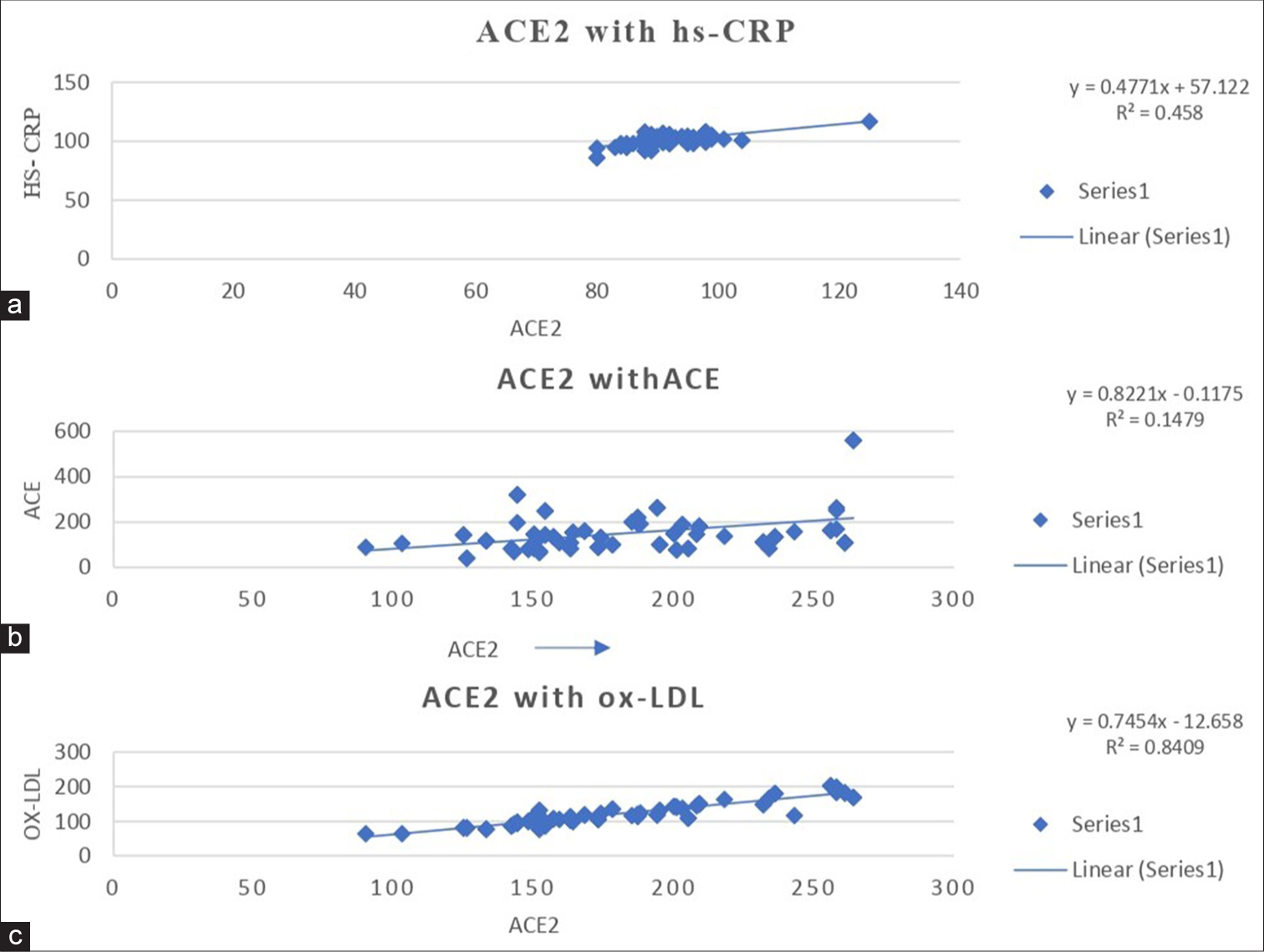
- Linear regression analysis of (a)ACE2 with hs-CRP, (b)ACE2 with ACE, and (c)ACE2 with ox-LDL levels in Type 2 diabetes mellitus with coronary heart disease subjects. ACE: Angiotensin converting enzyme, Ox-LDL: Oxidized-low-density lipoprotein, DM: Diabetes mellitus, CHD: Coronary heart disease, hs-CRP: High-sensitivity C-reactive protein.
DISCUSSION
The major finding of the study was that increased plasma ACE2 is associated with worse outcomes in patients with T2DM and increases the risk of CVD.[10] ACE2 is an extracellular enzyme expressed in different organs, including the pancreas. It exists in islets of the pancreas and is expressed by β - cells. ACE2 was found to regulate glucose homeostasis by activating the RAS and protecting the islet function of β-cells.[11] In diabetes, ACE’s hyperactivity results in more Ang II production than Ang (1–7) from Ang II by ACE2. Ang II causes endothelial dysfunction, platelet aggregation, and myocardial infarction.[4] Also, the ACE2 is released into circulation by the TACE and increases tissue Ang II levels while decreasing Ang (1–7) levels. The shedding of ACE2 from tissues into circulation, resulting in increased circulating ACE2 activity, has been proposed as a potential marker for CVD.[12]
The imbalance in the ACE/ACE2 ratio has been implicated in various pathological conditions, correlating with worse clinical outcomes. Specifically, ACE2 contributes to an elevated ACE/ACE2 ratio, which has been observed in CVD.[13] In our study, the ACE/ACE2 ratio increased in patients with diabetes, as well as in those with coexisting CHD. Another study reported that increased ACE2 activity enhanced the compensatory mechanism to produce more Ang (1–7), thereby increasing in ACE2/Ang (1–7) axis. The imbalance between ACE/Ang II and ACE2/Ang (1–7) may increase the risk of worse outcomes in group III. Therefore, the understanding of the molecular mechanism of the ACE/ACE2 ratio in T2DM is generally urgent and necessary to design some truly effective therapies.[14] This dysregulation suggests that the altered balance between ACE and ACE2 may play a critical role in the pathophysiology of these conditions, potentially serving as a biomarker for disease severity and progression. This strongly supports a key clinical role of ACE2 in CVD that was secondary to diabetes.
ACE2 showed a positive correlation with FBG, ACE, oxLDL, hs-CRP and ACE/ACE2 ratio. The study also found that group-III had significantly higher plasma ACE2 levels compared to group-I. A similar study was also reported study also found that ACE2 was positively correlated with FBG, hs- CRP in group-II,[15] which may be increased expression of ACE2 in islets tissue leading to the production of Ang 1–7. Increased ace2 expression is mainly regulated by blood glucose. If blood glucose is not effectively controlled with aggravation, glucose metabolism disorder and insulin resistance release more inflammatory mediators like hs-CRP is largely caused by repeated fluctuation of blood glucose.[2]
The result of the study found that Plasma ACE levels were highly significant in Group- III than compared to Group- II and GROUP-I. Another study also reported that Overexpression of ACE results in the shedding of ACE2 from pancreatic β-cell, which may increase Ang II in pancreatic islets. Angiotensin II has been found to affect the structural integrity of blood vessels by influencing the formation and breakdown of the extracellular matrix and increasing lipid oxidation.[16]
The result was previous findings that high plasma ACE2 is mainly affected by inflammatory mediators like hs-CRP and ox-LDL caused by repeated blood glucose fluctuation.[17] Additionally, ox-LDL attracts monocytes and cytokine cells, which leads to the accelerated formation of inflammatory cells.[18] The overexpression of ACE2 along with ox-LDL weakens the anti-inflammatory impact and makes the inflammatory effect more prominent, which may induce an ‘inflammatory factor storm’.[19] The results suggest that increased plasma ACE2 reflects underlying atherosclerosis rather than acute myocardial injury.
The results suggest that increased plasma ACE2 reflects underlying atherosclerosis rather than acute myocardial injury. Recent investigations demonstrated that the elevated level of plasma ACE2 was also connected to a greater risk of atrial fibrillation, and death was detected in Group- II patients.[20,21] So, investigations on the changes in the levels of ACE2 in T2DM and the source of inflammation are rarely reported. The study found a significant association between increased plasma ACE2 activity and adverse outcomes in patients with T2DM with CHD.
The limitation of the study is the small sample size, which further needs to be validated for large sample studies. The results shown may only apply to plasma and might not represent the amounts in other solid tissues or membrane-bound ACE2 receptors. So, the study was carried out in Group II and Group III for a better understanding of the mechanism, particularly in the high-risk groups. This study has identified ACE2 as a potential marker of CVD in T2DM and possibly a target for therapeutic intervention.
CONCLUSION
The study concludes that increased ACE2 levels are associated with the risk of CVD in T2DM.
Acknowledgment
The authors acknowledge the Department of Medicine, Cardiology and Master Health check-up unit of SRM Medical College Hospital and Research Centre, SRMIST, for the permitting and support.
Ethical approval
The research/study was approved by the Institutional Review Board at SRM Medical College Hospital & Research Centre, number 8717/IEC/2023, dated 8th November 2023.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Myocardial ischemia, carotid, and peripheral arterial disease and their interrelationship in type 2 diabetes patients. J Nucl Cardiol. 2009;16:878-87.
- [CrossRef] [PubMed] [Google Scholar]
- Changes of ACE2 in different glucose metabolites and its relationship with COVID-19. Medicine. 2022;101:e31102.
- [CrossRef] [PubMed] [Google Scholar]
- Angiotensin-converting enzyme 2: SARSCoV-2 receptor and regulator of the renin-angiotensin system: Celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126:1456-74.
- [CrossRef] [PubMed] [Google Scholar]
- ACE2-angiotensin-(1-7)-Mas axis and oxidative stress in cardiovascular disease. Hypertens Res. 2011;34:154-60.
- [CrossRef] [PubMed] [Google Scholar]
- ACE/ACE2 ratio: A key also in 2019 coronavirus disease (Covid-19)? Front Med. 2020;7:335.
- [CrossRef] [PubMed] [Google Scholar]
- Aldosterone and angiotensin: Role in diabetes and cardiovascular diseases. Eur J Pharmacol. 2012;697:1-2.
- [CrossRef] [PubMed] [Google Scholar]
- An insight into the mechanisms of COVID-19, SARS-CoV2 infection severity concerning β-cell survival and cardiovascular conditions in diabetic patients. Mol Cell Biochem. 2022;477:1681-95.
- [CrossRef] [PubMed] [Google Scholar]
- Roles of hepatic stellate cells in NAFLD: From the perspective of inflammation and fibrosis. Front Pharmacol. 2022;13:958428.
- [CrossRef] [PubMed] [Google Scholar]
- Role of cigarette smoking on serum angiotensin-converting enzyme and its association with inflammation and lipid peroxidation. Cureus. 2022;14:e27857.
- [CrossRef] [Google Scholar]
- Evaluation of pathophysiological relationships between renin-angiotensin and ACE-ACE2 systems in cardiovascular disorders: From theory to routine clinical practice in patients with heart failure. Crit Rev Clin Lab Sci. 2021;58:530-45.
- [CrossRef] [PubMed] [Google Scholar]
- The interaction of RAAS inhibitors with COVID-19: Current progress, perspective and future. Life Sci. 2020;257:118142.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 in relation to hyperglycemia and diabetes mellitus. Front Cardiovasc Med. 2021;8:644095.
- [CrossRef] [PubMed] [Google Scholar]
- High-salt diet increases glomerular ACE/ACE2 ratio leading to oxidative stress and kidney damage. Nephrol Dial Transplant. 2012;27:1793-800.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 and diabetes: A comprehensive review of angiotensin converting enzyme 2, mutual effects and pharmacotherapy. Front Endocrinol. 2021;12:772865.
- [CrossRef] [PubMed] [Google Scholar]
- Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17:122.
- [CrossRef] [PubMed] [Google Scholar]
- Angiotensin-converting enzyme 2 (ACE2) levels in relation to risk factors for COVID-19 in two large cohorts of patients with atrial fibrillation. Eur Heart J. 2020;41:4037-46.
- [CrossRef] [PubMed] [Google Scholar]
- The role of lipids and lipoproteins in atherosclerosis In: Endotext. South Dartmouth, MA: MDText.com, Inc.; 2019.
- [Google Scholar]
- A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:e1-9.
- [CrossRef] [PubMed] [Google Scholar]
- Role of the renin-angiotensin-aldosterone system and inflammatory processes in the development and progression of diastolic dysfunction. Clin Sci. 2009;116:467-77.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanisms of oxidized LDL-mediated endothelial dysfunction and its consequences for the development of atherosclerosis. Front Cardiovasc Med. 2022;9:925923.
- [CrossRef] [PubMed] [Google Scholar]
- Sustained hyperglycemia and its relationship with the outcome of hospitalized patients with severe COVID-19: Potential role of ACE2 upregulation. J Pers Med. 2022;12:805.
- [CrossRef] [PubMed] [Google Scholar]







