Translate this page into:
Comparative study for safety and efficacy of a novel folic acid formulation gel with 0.1% triamcinolone acetonide paste in experimentally induced oral ulcer
*Corresponding author: Sulthan Al Rashid, Department of Pharmacology, Faculty of Medicine, Bhaarath Medical College and Hospital, Selaiyur, Chennai, Tamil Nadu, India. sulthanalrashid@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Al Rashid S, Rahman SZ, Khan S. Comparative study for safety and efficacy of a novel folic acid formulation gel with 0.1% triamcinolone acetonide paste in experimentally induced oral ulcer. Indian J Physiol Pharmacol 2021;65(1):39-44.
Abstract
Objectives:
The objective of the present study was to evaluate the efficacy and safety of 0.1% novel folic acid gel in blunt dental instrument-induced oral ulcer in animal models (both rats and rabbits).
Materials and Methods:
The study was conducted in the Experimental Laboratory, Department of Pharmacology, Jawaharlal Nehru Medical College, Aligarh Muslim University. Animals were randomly divided into three interventional groups, namely Group 1 (folic acid gel), Group 2 (triamcinolone oromucosal paste) and Group 3 (control). We created a linear oral ulcer of 5 mm with the blunt dental instrument in both rats and rabbits. Each group consists of 10 animals (6 rats + 4 rabbits). Group 1 received test drug 0.1% novel folic acid gel formulated by us, Group 2 was positive control and received standard local drug 0.1% Kenacort triamcinolone oromucosal paste and Group 3 served as a negative control group where no treatment was given.
Results:
No untoward reaction was observed on the irritancy test. Kruskal–Wallis test applied for comparison of results. After 1 week of post-ulcer induction, there was a marked improvement in healing of oral ulcers in both Group 1 and Group 2, but more statistically significant healing was seen in Group 1 (folic acid gel). Both Groups 1 and 2 showed steady improvement in the healing of oral ulcers as compared to their pre-treated values and again were more marked in Group 1.
Conclusion:
Local folic acid gel supplementation was safe and had shown to augment oral ulcer healing in rats and rabbit’s animal models.
Keywords
Folic acid
Local drug delivery
Oral wound healing
Oral ulcer
INTRODUCTION
Oral ulceration is the discontinuation of oral epithelium due to many reasons such as trauma, infections, immunological reactions, nutritional deficiency and neoplastic disorders.[1] Folic acid is one of the Vitamin B, which is not synthesised de novo in humans and is obtained either through diet or synthesised by microflora in the gut. It has been established that deficiency of folic acid causes megaloblastic anaemia[2] and newborn neural tube defects in pregnancy.[3] Studies also suggest that folic acid deficiency can lead to the development of cancer, especially of the cervix.[4] Folic acid deficiency has also been associated with abnormalities in epithelial cells which are rapidly proliferating, especially oral cells. It has been shown that folic acid supplementation increases the resistance of gingiva to local irritants and decreases gingival inflammation.[5] The oral ulcer is normally treated with corticosteroids.[6] However, the therapeutic use of corticosteroids like triamcinolone for oral ulcers causes mucosal thinning and oral candidiasis.[7] Therefore, there is a need for drugs to manage oral ulcerations and desquamations with good efficacy and potency with minimal or no side effects.
Topical corticosteroids (TCs) remain the mainstay of treatment for oral ulcers.[6] An array of diverse TCs is available. TCs decrease painful symptoms but not the rate of ulcer relapse. One of the frequently used preparations is triamcinolone acetonide in carboxymethyl cellulose paste. The notable side effects of corticosteroids are oral candidiasis, hypogeusia, drug hypersensitivity, secondary adrenal insufficiency, hypothalamic-pituitary-adrenal axis inhibition and central serous chorioretinopathy.[8] Therefore, there is a need for better topical agents for the treatment of oral ulcers, which could prove rational and more efficacious in treating oral ulcers with minimal or no side effects, when compared to corticosteroids. As oral administration of vitamins is accompanied by positive oral mucosal health, great therapeutic index and minimal side effects,[9] Vitamin B9 or folic acid has shown to play a fairly positive role as far as gingival and oral mucosal health is concerned.[5] The gel form of folic acid as a local application has not been ever tried. In the light of the above background, we propose to use novel folic acid gel (0.1%) formulated at our pharmacy division of Ajmal Khan Tibbiya College, as a topical agent for the healing of experimentally induced ulcers in animal models and also to compare it with standard topical steroid therapy.
MATERIALS AND METHODS
Animal procedures and experimental protocols were approved by the Ethical Review Board of Institutional Animal Ethical Committee of Jawaharlal Nehru Medical College, Aligarh Muslim University, Aligarh (Registration No: 401/GO/Re/S/2001/CPCSEA), and the study was carried out as per ARRIVE (Animal Research: Reporting of in vivo experiments) guidelines.
Preparation 100 ml of 0.1% folic acid gel
The following constituents were used in making 0.1% folic acid gel: Carbopol 934 CDH – 0.5 g, polyethylene glycol 200 – 15 ml, glycerine – 5 ml, methyl paraben – 0.18 g, propylparaben – 0.02 g, aspartame – 0.4 g, folic acid – 0.1 g and distilled water – 78.8 ml.[10] The above constituents were mixed gently by adding them in a glass jar gently stirring on a magnetic stirrer until a gel form appeared. The gel thus formed was stored in plastic bottles in a refrigerator at −5°C at the Department of Saidla (Unani Pharmacy, Ajmal Khan Tibbiya College, Aligarh Muslim University, Aligarh) [Figure 1].
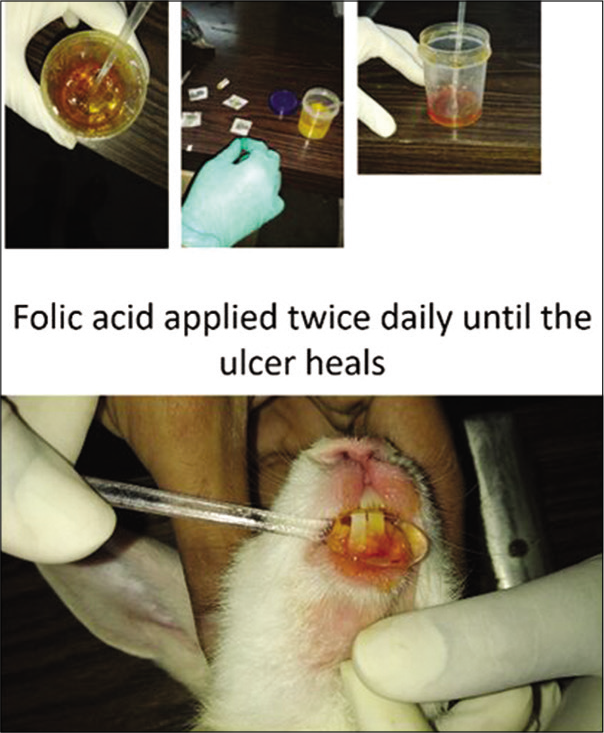
- Folic acid in the gel form. The folic acid gel was applied twice daily until the oral ulcer heals in both rabbits and rats.
Study design
The study was conducted in the Experimental Laboratory of the Department of Pharmacology in collaboration with the Department of Periodontics and Community Dentistry, Dr. Z. A. Dental College, AMU, Aligarh.
Animal models
We used the following animals in our study:
Rats (total 18 albino rats of both sexes weighing 150–250 g)
Rabbits (total 12 rabbits of both sexes weighing 400– 1000 g).
The total sample size is 30 animals divided into three groups. Each group consisted of six rats and four rabbits.
The animals were obtained and kept in cages under standard environmental conditions (22°C ± 3°C, 12 h light/dark cycle) at the animal house of Jawaharlal Nehru Medical College, Aligarh Muslim University. All animals had access to standard laboratory pellets and water ad libitum before and during the experiment.
Drugs
The novel folic acid gel 0.1% was synthesised at the Department of Saidla, Ajmal Khan Tibbiya College, while the 0.1% triamcinolone oromucosal (Kenacort) paste was procured from the local market.
The animals in all three groups were anesthetised by pentobarbitone (rabbits: 40 mg/kg IV and rats: 50 mg/kg IP). A linear traumatic oral ulcer of 5 mm was induced in rabbits and rats by a blunt dental instrument on the gums. The tests animals randomly allocated into three test arms as follows;
Group 1
This group consisted of six rats and four rabbits and was treated with a test drug (0.1% novel folic acid gel) on the oral ulcers. Novel 0.1% folic acid gel administered topically on the induced ulcer site twice daily (morning 8 am and evening 4 pm) for 14 days.
Group 2
This group consisted of six rats and four rabbits treated with 0.1% triamcinolone acetonide (Kenacort) oral paste on the induced oral ulcers. Triamcinolone acetonide 0.1% gel (Kenacort) was applied topically twice daily (morning 8 am and evening 4 pm) for 14 days.
Group 3
This was the control group having six rats and four rabbits and was treated with distilled water on the induced oral ulcers. Distilled water was applied twice daily (morning 8 am and evening 4 pm) for 14 days.
The ulcer healing was checked from baseline to the 1st week and 2nd week, the diameter of the oral ulcers was measured using millimetre calibrated periodontal probe at baseline 0, 7 and 14 days in the respective treatment arms.[11]
Testing the drug safety on intact skin
To test the drug toxicity and local irritancy, animals were anesthetised by pentobarbitone (rabbits: 40 mg/kg IV and rats: 50 mg/kg IP) and fixed to an animal board. The anterior aspect of the animal shaved and circular areas of 2.5 cm in diameter were drawn on the animal’s abdomen. Four areas were inscribed for rats and eight areas for rabbits. About 20% aqueous formaldehyde solution (0.1 ml) was then painted on the circled areas and allowed to evaporate, and the same repeated 3 times. Following this, gel formulation of a drug impregnated on 2.5 cm circular funnel pads and these pads applied to the areas previously treated with formaldehyde. After placing the pads on the skin, a polythene sheet was placed over the whole area. The axilla of the animal is then injected with 0.5–2 ml of trypan solution blue (0.5%). Sixteen hours later, the funnel pads were detached, and toxicity was estimated by the trypan blue accumulation at the site where the drug was applied.[12]
Statistical analysis
Data were analysed using IBM SPSS statistics 20 software. Kolmogorov–Smirnov and Shapiro–Wilk tests were performed to check the normality of the data which came out to be significant, implying the data to be not normally distributed suggesting a non-parametric test (Kruskal–Wallis test) for comparison of reduction in ulcer size among all the three groups.
RESULTS
We compared the mean reduction in oral ulcer size in rabbit and rat models receiving novel folic acid gel (Group 1), triamcinolone oromucosal paste (Group 2) and control group without receiving any treatment (Group 3).
In rabbit models
At the 1st week of treatment [Figures 2-4], rabbits belonging to the topical folic acid gel group showed a greater reduction in mean oral ulcer size [Table 1 and Figure 5] and exhibited a significant maximum mean reduction in oral ulcer size from baseline (5 mm) (P < 0.05) [Table 2] as compared to triamcinolone and control groups.
| Groups | Baseline | 1 week | 2 weeks |
|---|---|---|---|
| Folic acid gel | 5±0 | 0.5±0.5 | 0 |
| Triamcinolone paste | 5±0 | 2±0 | 0 |
| Control | 5±0 | 2.75±0.43 | 0 |
| Groups | Baseline to 1 week | Baseline to 2 weeks |
|---|---|---|
| Folic acid gel | (4.5±0.5)* | 5±0 |
| Triamcinolone paste | 3±0 | 5±0 |
| Control | 2.25±0.43 | 5±0 |
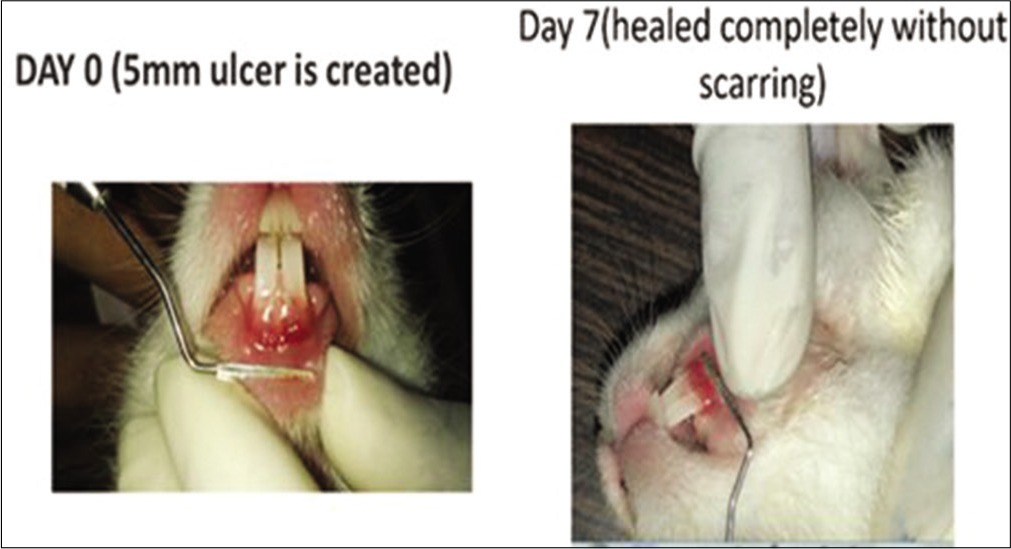
- Local folic acid gel application in the rabbits. On day 0, a 5 mm traumatic ulcer was induced in this rabbit belonging to the folic acid gel group. On day 7, the ulcer was completely healed with primary intention without scarring.
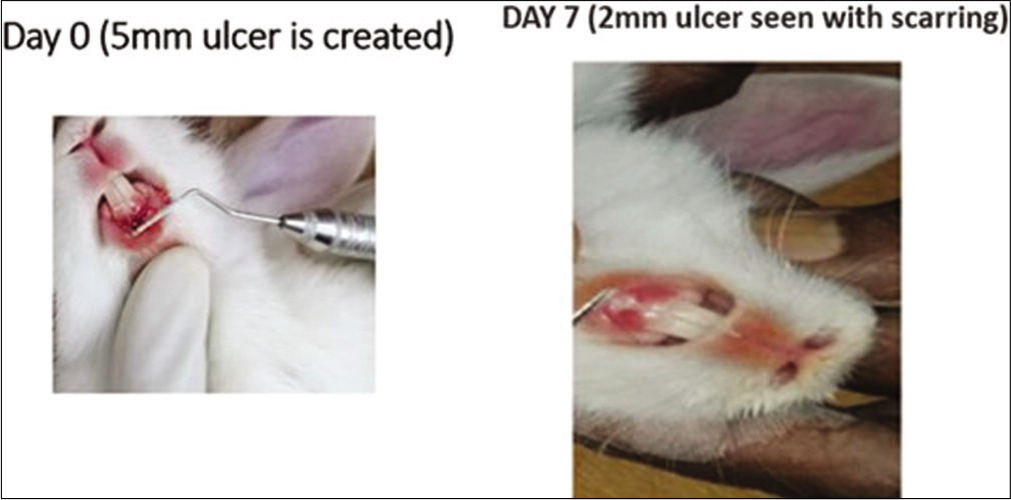
- Local triamcinolone oromucosal paste application in the rabbits. On day 0, a 5 mm traumatic ulcer was induced in this rabbit belonging to the triamcinolone paste group. On day 7, the ulcer size reduced to 2 mm with scarring. By the 2nd week, ulcer completely healed with scarring without primary intention.
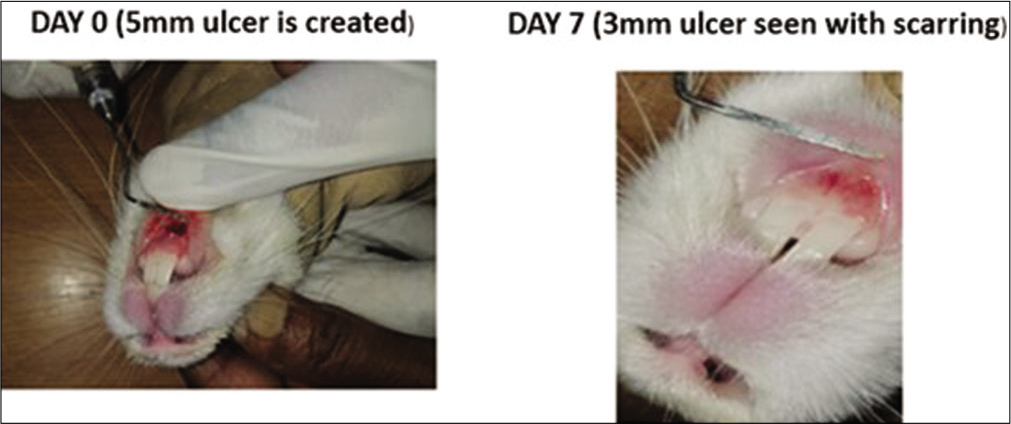
- Control group in rabbits. On day 0, a 5 mm traumatic ulcer was induced in this rabbit belonging to the control group. On day 7, the ulcer size reduced to 3 mm with scarring. By the 2nd week, ulcer completely healed with scarring without primary intention.
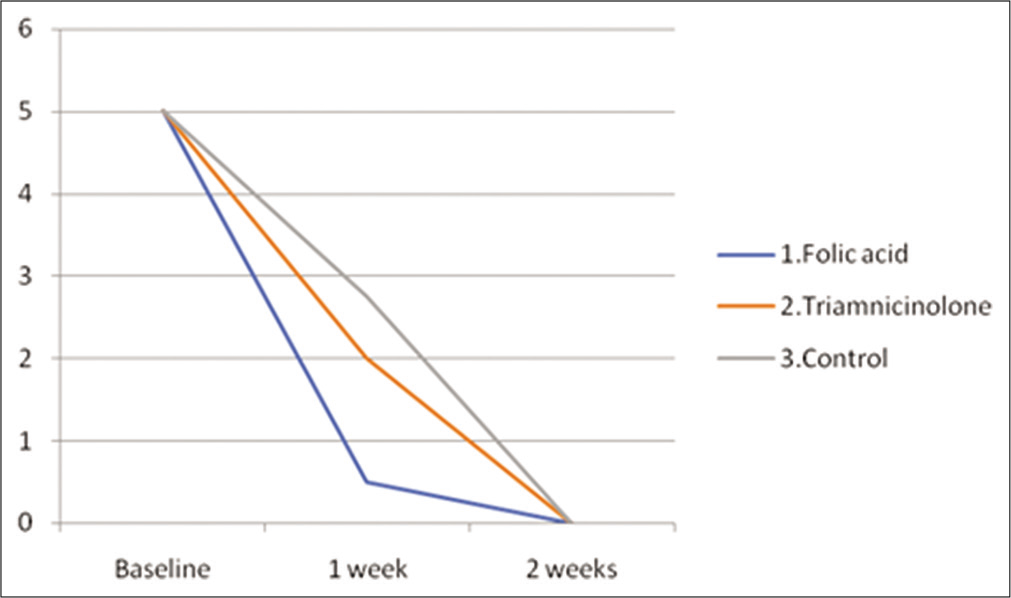
- Mean ulcer size (mm) in all the three intervention groups at various time points (baseline, 1 week and 2 weeks) in rabbits.
However, by the end of the 2nd week, rabbits belonging to all three groups showed complete healing [Table 1 and Figure 5] by exhibiting a maximum mean reduction in oral ulcer size from baseline (P > 0.05) [Table 2].
In rat models
At the 1st week of treatment [Figures 6-8], rats belonging to the topical folic acid gel group showed a greater reduction in mean oral ulcer size [Table 3 and Figure 9] and exhibited a significant maximum mean reduction in oral ulcer size from baseline (P < 0.05) [Table 4] as compared to triamcinolone and control groups.
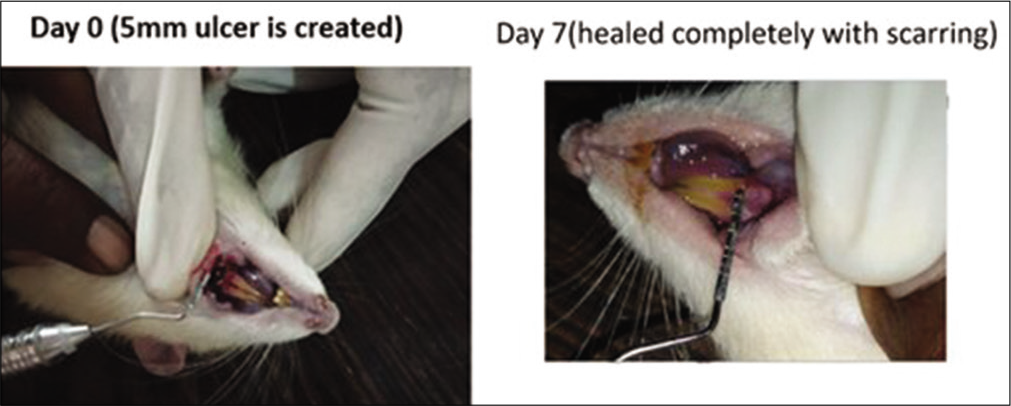
- Local folic acid gel application in the rats. On day 0, a 5 mm traumatic ulcer was induced in this rat belonging to the folic acid gel group. On day 7, the ulcer was completely healed with scarring without primary intention.
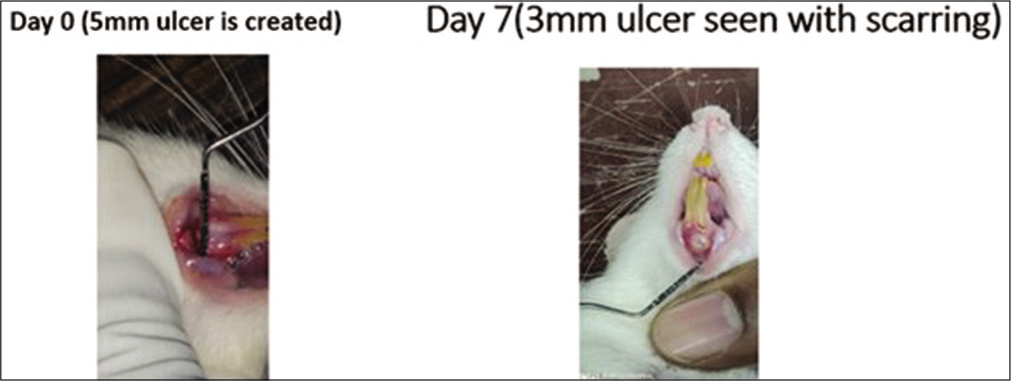
- Local triamcinolone oromucosal paste application in the rats. On day 0, a 5 mm traumatic ulcer was induced in this rat belonging to the triamcinolone paste group. On day 7, the ulcer size reduced to 3 mm with scarring. By the 2nd week, ulcer completely healed with scarring without primary intention.
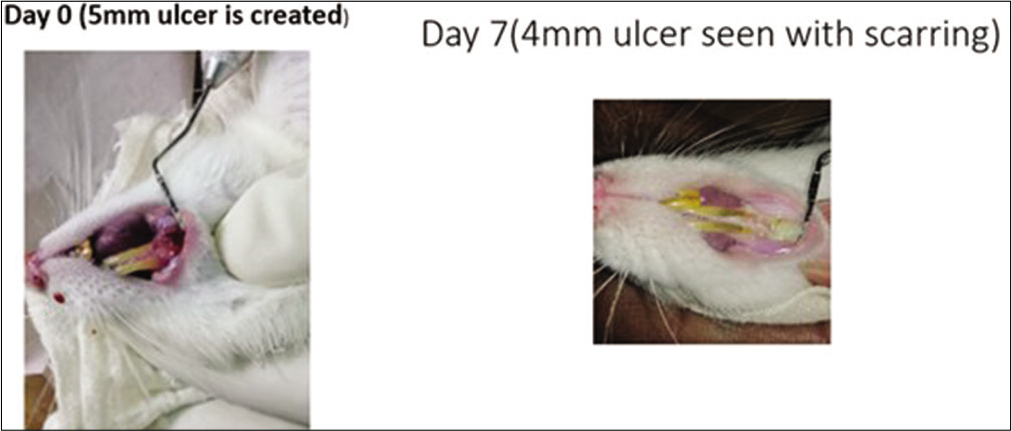
- Control group in the rats. On day 0, a 5 mm traumatic ulcer was induced in this rat belonging to the control group. On day 7, the ulcer size reduced to 4 mm with scarring. By the 2nd week, ulcer completely healed with scarring without primary intention.
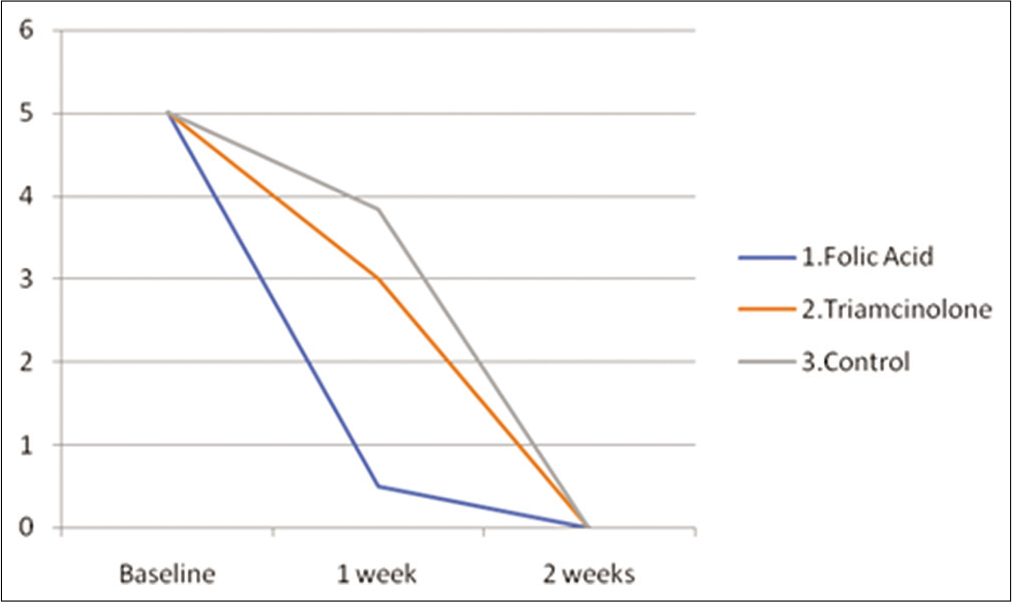
- Mean ulcer size (mm) in all the three intervention groups at various time points (baseline, 1 week and 2 weeks) in the rats.
| Groups | Baseline | 1 week | 2 weeks |
|---|---|---|---|
| Folic acid gel | 5±0 | 0.5±0.5 | 0 |
| Triamcinolone paste | 5±0 | 3±0 | 0 |
| Control | 5±0 | 3.83±0.37 | 0 |
| Groups | Baseline to 1 week | Baseline to 2 weeks |
|---|---|---|
| Folic acid gel | (4.5±0.5)* | 5±0 |
| Triamcinolone paste | 2±0 | 5±0 |
| Control | 1.2±0.4 | 5±0 |
However, by the end of the 2nd week, rats belonging to all three groups showed complete healing [Table 3 and Figure 9] by exhibiting a maximum mean reduction in oral ulcer size from baseline (P > 0.05) [Table 4].
DISCUSSION
Folic acid is available in various delivery forms such as tablets, capsules and injections. The use of topical folic acid gel is an innovative novel application in traumatic oral ulcers as a local targeted drug delivery system. Our novel topical folic acid gel is a new compound that has been prepared for the 1st time. The topical folic acid gel provides the additional advantage of drug delivery to the local ulcer site, thereby increasing the bioavailability by bypassing the first-pass metabolism in the liver and circumventing the systemic side effects of oral and injectable therapy.
A linear traumatic oral ulcer of 5 mm was created with a blunt dental instrument on the gums of both rats and rabbits. The healing of the ulcers was assessed at the 1st and 2nd week in our experimental studies to study and compare the efficacy of topical folic acid gel and triamcinolone oromucosal paste on healing outcomes. We assigned three groups in our study. Each group consisted of 10 animals (six rats and four rabbits). The first group for our test drug topical folic acid gel, the second group for positive control with standard drug 0.1% Kenacort triamcinolone oromucosal paste and the third group as a negative control group where no treatment was given. Novel topical folic acid gel and triamcinolone oromucosal paste were applied over the oral ulcer in their respective group 2 times daily for 14 days. At the end of the 7th day and 14th day, the size of the oral ulcer was measured with periodontal probe calibrated with markings from 0 to 10 mm.
In rabbit models, we compared the mean reduction in oral ulcer size from baseline to 1 week and 2 weeks in all the three groups using the Kruskal–Wallis test. Group 1 shows maximum healing among all three groups in the 1st week (P < 0.01) [Table 2]. Hence, we can confirm that rabbits belonging to the topical folic acid gel group showed faster healing of the traumatic oral ulcer within the 1st week as compared to other groups. However, the mean reduction in ulcer size from baseline to 2 weeks was the same in all the three groups (P > 0.05) [Table 2] which implies that the traumatic ulcers in rabbits of all the three groups were completely healed by the 2nd week of treatment (P > 0.05).
Like in rabbit models, we compared the mean reduction in oral ulcer size in rat models from baseline to 1 week and 2 weeks in all the three groups using the Kruskal–Wallis test. Group 1 shows maximum healing among all three groups (P < 0.001) in the 1st week [Table 4]. Hence, we can conclude that rats belonging to the topical folic acid gel group showed faster healing of the traumatic oral ulcer within the 1st week as compared to other groups.
However, the mean reduction in ulcer size from baseline to 2 weeks was the same in all the three groups (P > 0.05) [Table 4] which implies that traumatic ulcers in the rats of all the three groups were completely healed by the 2nd week (P > 0.05).
Furthermore, three rats in the triamcinolone group showed bleeding in the oral ulcer site during the course of healing at the end of the 1st week, which may be its side effect of delayed wound healing. However, folic acid gel treated rat models had better and faster healing outcomes as compared to the triamcinolone group. Interestingly, all the animals (rabbits and rats) healed with scarring except the rabbits belonging to the topical folic acid gel group which healed with primary intention without scarring because of thick oral mucosa and well-developed oral vasculature as compared to the rats.
Our study is also comparable to another study, where the usage of 5 ml (5 mg in 5 ml) folate mouthwash rinse twice daily for 4 weeks for 1 min on confirmed gingivitis in non-pregnant adults led to better gingival health.[13] These evidences show the beneficial effect of folic acid in the management and maintenance of oral and periodontal health. The novelty of our study compared with other studies is that we for the 1st time used topical folic acid in the local drug delivery gel form for the treatment of traumatic oral ulcers in animal models and we demonstrated its efficacy and safety in the treatment of traumatic ulcers induced in animal models.
In conclusion, our study revealed better healing outcomes with topical folic acid gel without any local or systemic side effects. Our novel topical folic acid gel showed faster healing of the traumatic oral ulcer by the end of the 1st week as compared to triamcinolone paste and control groups in both the rabbits and rat’s model. Furthermore, our study is comparable to the earlier study, where dietary supplementation of 2 mg systemic folic acid revealed an upsurge in the resistance of gingival epithelial cells to the local irritants and thus led to a diminution in inflammation over a period of 30 days.[14]
The limitation of the study is the limited sample size (12 rabbits and 18 rats), it needs further exploration in terms of pharmaceutical stability and penetration data, and most importantly, we need to test the efficacy and safety in humans using clinical trials/interventional studies for the management of oral mucosal diseases.
CONCLUSION
Folic acid supplementation positively influences the management and maintenance of periodontal and oral mucosal health. Topical folic acid gel supplementation could be more efficacious and safer in the healing of oral ulcers than corticosteroids as found in our study. Clinical trials using 0.1% novel folic acid gel could further be conducted to prove its efficacy and safety on humans.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Recurrent aphthous stomatitis. Dent Clin North Am. 2014;58:281-97.
- [CrossRef] [PubMed] [Google Scholar]
- Megaloblastic anemia and other causes of macrocytosis. Clin Med Res. 2006;4:236-41.
- [CrossRef] [PubMed] [Google Scholar]
- Periconceptional folate deficiency and implications in neural tube defects. J Pregnancy. 2012;2012:295083.
- [CrossRef] [PubMed] [Google Scholar]
- Association between folate status and cervical intraepithelial neoplasia. Eur J Clin Nutr. 2016;70:837-42.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of topical application of folic acid on gingival health. J Oral Med. 1978;33:22-2.
- [Google Scholar]
- The treatment of chronic recurrent oral aphthous ulcers. Dtsch Arztebl Int. 2014;111:665-73.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and radiological assessment of effects of long-term corticosteroid therapy on oral health. Dent Res J (Isfahan). 2013;10:666-73.
- [Google Scholar]
- A potential side effect of oral topical steroids: Central serous chorioretinopathy. Indian J Dent Res. 2018;29:107-8.
- [CrossRef] [PubMed] [Google Scholar]
- Folic acid safety and toxicity: A brief review. Am J Clin Nutr. 1989;50:353-8.
- [CrossRef] [PubMed] [Google Scholar]
- A novel formulation of Folic acid gel in the treatment of desquamative gingivitis. Bangladesh J Med Sci. 2020;19:5-6.
- [CrossRef] [Google Scholar]
- Experimental model of traumatic ulcer in the cheek mucosa of rats. Acta Cir Bras. 2011;26:227-34.
- [CrossRef] [PubMed] [Google Scholar]
- Folate mouthwash: Effects on established gingivitis in periodontal patients. J Clin Periodontol. 1984;11:619-28.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of folic acid on gingival health. J Periodontol. 1976;47:667-8.
- [CrossRef] [PubMed] [Google Scholar]






