Translate this page into:
Effect of acute bout of moderate exercise on P300 component of event-related potential in young women during different phases of menstrual cycle: A pilot study
*Corresponding author: Manisha Kar, Department of Physiology, AIIMS Bhubaneswar, Sijua, Patrapara, Bhubaneswar, Odisha, India. physio_manisha@aiimsbhubaneswar.edu.in
-
Received: ,
Accepted: ,
How to cite this article: Challawar R, Menon A, Kar M, Mahapatra SC. Effect of acute bout of moderate exercise on P300 component of event-related potential in young women during different phases of menstrual cycle: A pilot study. Indian J Physiol Pharmacol 2020;64(4):272-8.
Abstract
Objectives:
Ovarian hormones not only regulate reproductive functions but also are implicated in emotional and cognitive processing. But, menstrual cycle studies do not provide convincing evidences. However, evidence supports a link between estrogen depletion and risk for Alzheimer’s disease. Physical exercise has shown to improve a person’s attentiveness and cognitive skills. Since both exercise and ovarian hormones may affect cognition, the present study intends to determine the effect of acute bout of moderate exercise on cognitive processing in young women in early proliferative phase and mid-luteal phase.
Materials and Methods:
It was a cross-sectional, observational study conducted on young, normally menstruating women of 18-35 years (n = 20). Each participant attended two laboratory sessions, early follicular phase (initial 3 days post-menstruation) and mid luteal phase (days 21-24). The participants filled up Godin Leisure-Time Exercise Questionnaire (GLTEQ) and their basal central, brachial blood pressures and P300 Event Related Potential (ERP) were recorded. Then, the subjects performed step test till they achieved 60-80% of their maximum heart rate. Afterwards, their central and brachial blood pressure and heart rate were recorded. The participants then filled up Borg perceived exertion scale questionnaire. Post exercise P300 ERP was recorded after 15 min of exercise.
Results:
Significant changes in all cardiovascular parameters in post-exercise session were observed in both phases of menstrual cycle. The amplitude and latencies of P300 ERP showed no significant difference at resting state (pre-exercise) during both phases. P300 ERP latency showed significant decrease in post-exercise session when recorded at Cz (P = 0.024, P = 0.05) and Pz position (P = 0.03, P = 0.003) in both phases except in Fz position. But there was no significant change in amplitude. MANCOVA analysis revealed that only amplitude of P300 ERP (P = 0.023) in post-exercise session during mid-luteal phase was affected by basal activity level significantly.
Conclusion:
The present study documented that an acute bout of moderate exercise caused significant decrease in latency of P300 ERP in the participants during both phases of menstrual cycle. Therefore, it can be stated that even acute bout of moderate exercise significantly enhances attention allocation, working memory in the participants; thereby it enhances cognitive functioning of the individual.
Keywords
Exercise
P300 event-related potential
Cognition
Young women
Ovarian steroid hormones
INTRODUCTION
Mounting evidences suggest that ovarian hormones not only regulate reproductive functions and behaviour but also act as neurosteroids. Cognitive function refers to the brain’s ability to process information including attention, pattern recognition, learning, memory, problem solving, language processing and abstract reasoning.[1] Oestrogen and progesterone are implicated in emotional and cognitive processing as well.[2,3] These hormones have the ability to stimulate neuron outgrowth, synaptogenesis and dendritic branching in neuroplasticity.[4] Oestrogen-regulated synapse formation and turnover are mediated through both genomic and rapid, non-genomic mechanisms in cognitively relevant brain regions, such as hippocampus and prefrontal brain regions.[5] Progesterone receptors are also identified in cognitively relevant brain regions, including frontal cortex, hypothalamus, thalamus, hippocampus, amygdala and cerebellum.[6] Unopposed oestradiol administration can enhance prefrontal cognitive processing as evidenced by its positive effects on verbal working memory and attention.[7] However, menstrual cycle study results do not provide convincing evidence to support significant cognitive changes across menstrual cycle phase because of small effect size.[8] Moreover, there is a possibility that menstrual cycle related changes in cognition are subtle and so it may not be reflected in performance per se as studied earlier, but may influence cognitive strategies to achieve the goal.[9]
On the other hand, evidence supporting a link between oestrogen depletion and risk for Alzheimer’s disease (AD) appears relatively consistent.[10] High level of progesterone during luteal phase is also associated with impaired emotion recognition accuracy and enhanced emotional memory.[8] Neuroprotective effects of oestrogen are through modulation of molecules involved in apoptosis and its action as an antioxidant.[1] Altogether, these data support the pivotal role played by oestrogen and progesterone in cognitive processing.
Physical exercise has shown to improve a person’s attentiveness and cognitive skills. A vast literature supports the notion that monoamine systems, namely dopamine, norepinephrine and serotonin, hormones, namely endocannabinoids and brain-derived neurotropic factor (BDNF) act in a concerted fashion to enhance exercise-induced various aspects of cognitive function.[11] Several studies documented the effect of acute bout of moderate exercise on executive function of brain in pre-adolescent children and older adults. The studies related to the effect of the physical activity on the brain and cognition has grown in interest in recent years, with an increasing number of reports indicating that chronic participation and single, acute bouts of exercise, benefit a host of cognitive processes.[12] However, some studies fail to replicate the beneficial effect of acute exercise.[13] Moreover, fewer studies have investigated the effect of acute exercise on event-related potentials (ERPs), which are time-locked brain responses to specific events.[14] Since both exercise and ovarian hormones seem to be having an effect on cognition, the present study intends to determine the effect of acute bout of moderate exercise in cognitive processing in young women in early proliferative phase when the levels of oestrogen and progesterone are low and midluteal phase, when the levels of both the hormones are high.
MATERIALS AND METHODS
Participants
It was a cross-sectional observational study conducted on young, normally menstruating women with normal auditory capability of age group 18–35 years and without any major chronic illness and neurocognitive deficit (n = 20). However, data of 19 subjects were analysed because of inadequate data. The ethical approval for this study was obtained from Institute Ethics Committee, AIIMS, Bhubaneswar. The study was conducted in clinical physiology laboratory. Each participant attended two laboratory sessions, one of which was in the early follicular phase (initial 3 days post-menstruation) and the other was in mid-luteal phase (days 21–24). Phases were determined by taking menstrual history. Both sessions were attended preferably at the same time of the day. Written informed consent was obtained from all the participants before commencement of the study. The study protocol is depicted in [Figure 1].

- Schematic representation of the protocol of the present study.
Procedures
The participants were requested to refrain from tea, coffee at least 2 h before laboratory session. They were asked to fill up Godin Leisure-Time Exercise Questionnaire (GLTEQ) to understand their leisure time exercise habit.[15] The data collection pro forma was used to record information pertaining to anthropometric measurements, namely height, weight, body mass index (BMI), waist–hip ratio and detailed menstrual history, following which physiological parameters were recorded. Basal brachial artery systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured using mercury sphygmomanometer following standard protocol and mean arterial pressure (MAP), pulse pressure (PP) values were derived. Central BP and HR were recorded using USCOM BP+® (USCOM Ltd., Sydney, Australia). USCOM BP+® equipment employs suprasystolic oscillometric technology to compute central blood pressure. Following it, basal P300 was recorded. Afterwards, the subjects were asked to perform step test till they achieved 60–80% of their maximum heart rate (maximum heart rate = 220 – age) during exercise.[16] The heart rate of the subjects was monitored following exercise by pulse oximeter and P300 ERP was recorded when their heart rate returned to basal value. At the end of exercise, the participants filled up Borg perceived exertion scale questionnaire.
Recording and analysis of P300 ERP and stimuli
Event-related potentials (ERPs) were recorded using Neuropack X1 MEB-2300K (Nihon Kohden, Tokyo, Japan). Silver-silver chloride electrodes were placed on prescribed positions [A1, A2 (reference electrodes), FPz (ground electrode), Fz (medial frontal), Cz (medial central) and Pz (medial parietal) {active electrodes}] as per international 10–20 system on the subject’s scalp after proper abrasion of the desired locations on the scalp [Figure 2].
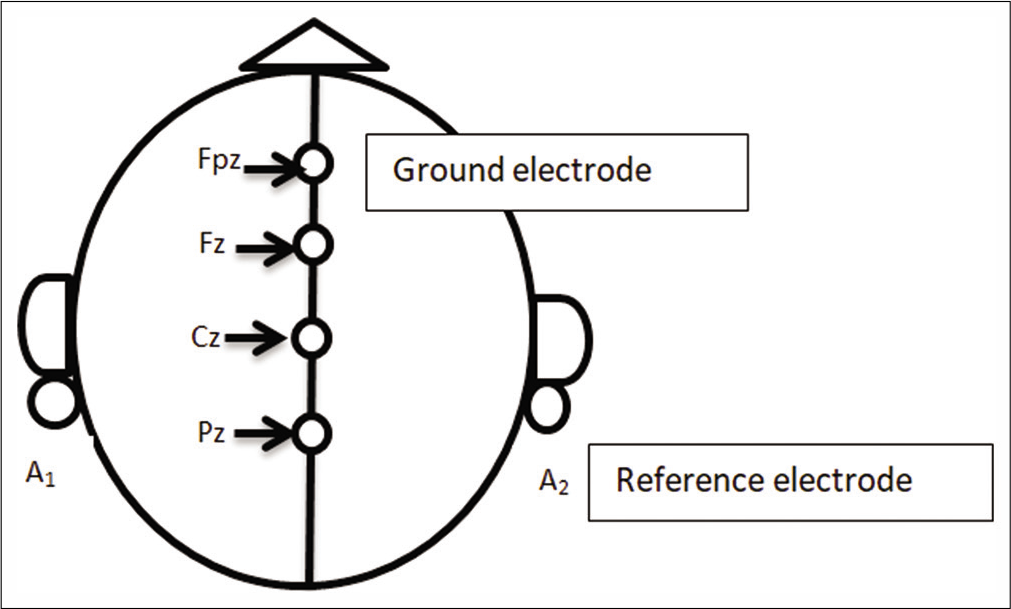
- Schematic representation of the placement of electrodes on the scalp as per international 10-20 system.
All the electrodes were connected to the designated slots in the jack box. The jack box was connected to amplifier of the recording instrument eventually. The impedances of all electrodes were kept below 5 kΩ. ERP signals were digitised at a sampling rate of 1000 Hz and were amplified (band pass, 0.1– 40 Hz). The participant was asked to use headphone as auditory stimuli were presented to her in ‘odd ball paradigm’ fashion. The subject was asked to respond to target auditory stimulus (40 dB at 2 KHz tone, 20% rare) in the background of non-target auditory stimuli (40 dB at 1 KHz tone, 80% frequent). These two auditory stimuli were presented to the participant at the rate of 0.5 Hz. The number of trials was 30 for each session. Finally, each trial waveform was averaged. A positive potential with its latency approximately 300 ms (200–400 ms) was scored as P300 ERP after the target stimulus, which the subject was directed to pay attention to. The amplitude of P300 wave was calculated between N200 and P300 peaks. The rejection rate of auditory stimulus during recording of P300 ERP was around <5% in pre- and post-exercise session irrespective of phase of cycle. [Figure 3a and b] illustrates the representative averaged P300 event-related potential recorded at Fz, Cz and Pz electrode positions in pre-exercise and post-exercise session, respectively.
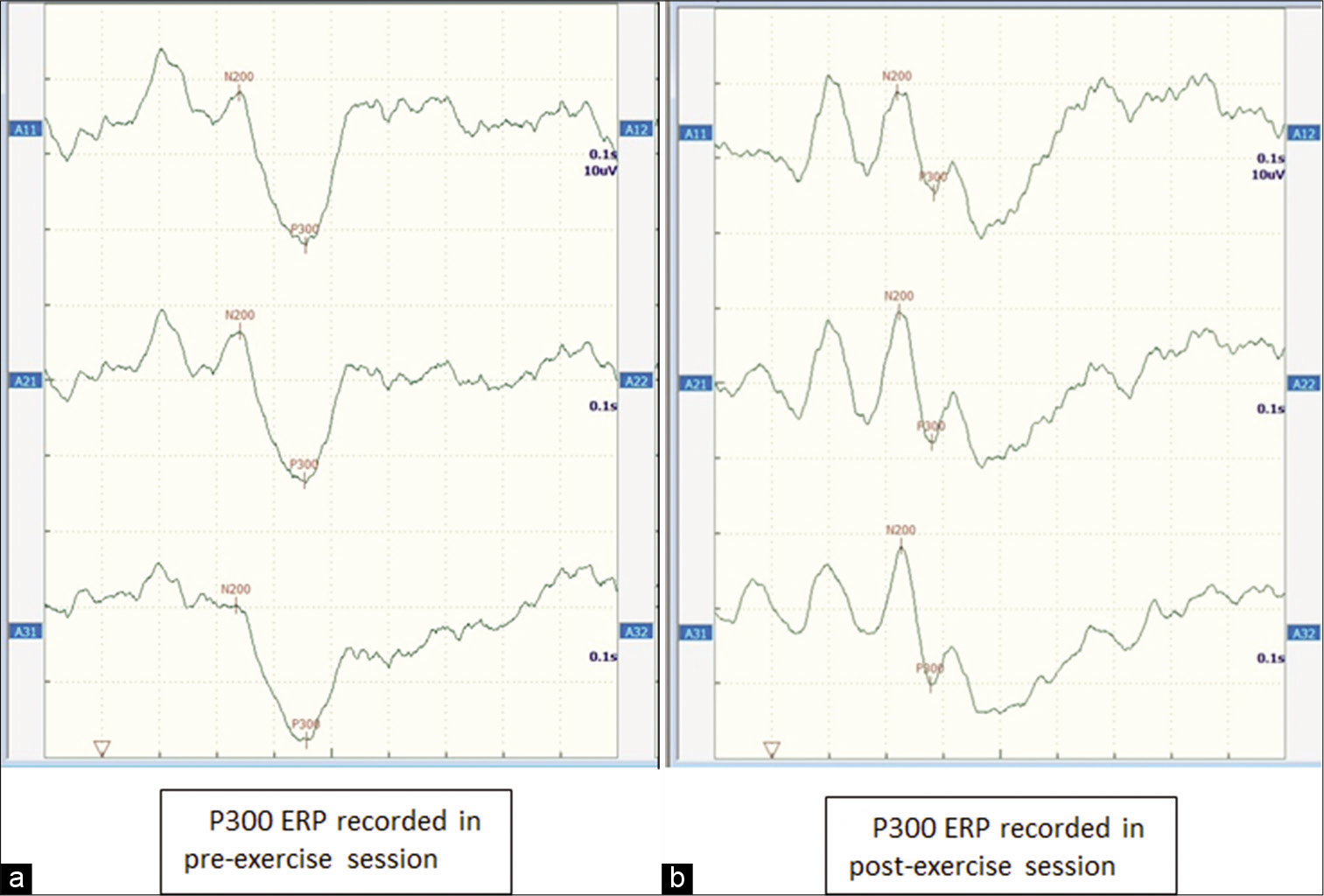
- Averaged P300 event-related potentials recorded at Fz (upper panel), Cz (middle panel) and Pz (lower panel) position, respectively. (a) Representative graph recorded at pre-exercise session and (b) shows representative graph recorded at post-exercise session.
Statistical analysis
The distribution pattern of data was tested by Shapiro– Wilk test of normality and it was found that data were not normally distributed. Therefore, the data are presented as median (interquartile range). Wilcoxon signed-rank test was employed for comparison of variables in pre- and post-exercise session during early follicular and mid-luteal phase. Wilcoxon signed-rank test was also employed to compare P300 ERP amplitude and latencies at resting state (pre-exercise) during early follicular and mid-luteal phase. MANCOVA has been employed to examine the effect of basal activity level on P300 ERP amplitude and latencies, while age, height, weight, BMI and WHR were considered as covariates in both phases. All statistical calculations have been done using computer programmes (Microsoft Excel 2013, Microsoft Corp., Redmond, WA) and statistical analysis has been done by SPSS 20 version (SPSS, IBM Inc., Chicago, IL). A two-tailed P < 0.05 has been taken as the cutoff level of significance.
RESULTS
A total of 20 subjects participated in the present study. However, the data of 19 subjects were analysed because of dropout. The basal characteristics of the study participants are presented in [Table 1].
| Age (year) [(median (IQR difference)] | Height (cm) [(median (IQR difference)] | Weight (kg)[(median (IQR difference)] | BMI (kg/m2) [(median (IQR difference)] | Waist–hip ratio (WHR) [(median (IQR difference)] |
|---|---|---|---|---|
| 20.0 (2.0) | 160.0 (12) | 55.0 (10) | 20.9 (2.9) | 0.8 (0.1) |
Data are presented as median (interquartile range)
It is evident from [Table 1] that the participants were mostly in the age group of 19–21 years. BMI of the subjects was mostly in between 19.4 and 22.13. Therefore, BMI of the participants was mostly normal as per the WHO Asian criteria. Waist–hip ratio (WHR) of the subjects was mostly little above normal range (normal being <0.8). Six participants were found to be active and 13 subjects were insufficiently active as per Godin Leisure-Time Exercise Questionnaire score (GLTEQ score).[15] The participants were asked to perform step test till they achieved 60–80% of their maximum heart rate.[16] The original Borg scale or category scale (6–20 scale) was used to analyse Borg perceived exertion scale questionnaire, which was administered at the end of step test. The rating of perceived exertion score (RPE score) is displayed in [Table 2].
| Rating | Early follicular phase | Mid-luteal phase |
|---|---|---|
| Extremely light | 01 | 01 |
| Very light | 05 | 05 |
| Light | 06 | 07 |
| Somewhat hard | 07 | 06 |
GLTEQ scoring was performed as per Godin Leisure-Time Exercise Questionnaire score[15]
It is evident from [Table 2] that most of the subjects perceived physical activity as light to extremely light excepting few subjects. The comparison of various physiological parameters is displayed in [Table 3].
| Variables | Phase | |||||
|---|---|---|---|---|---|---|
| Follicular | Luteal | |||||
| Pre-exercise | Post-exercise | Pvalue | Pre-exercise | Post-exercise | Pvalue | |
| Central systolic blood pressure (mmHg) | 98.0 (15) | 112.0 (10)**** | 0.0001 | 98.0 (15) | 110.0 (13)*** | 0.001 |
| Central diastolic blood pressure (mmHg) | 68.0 (13) | 75.0 (11)** | 0.005 | 66.0 (18) | 72.0 (10) | 0.100 |
| Peripheral SBP (mmHg) | 107.0 (13) | 121.0 (15)**** | 0.0001 | 107.0 (14) | 122.0 (19)* | 0.002 |
| Peripheral DBP (mmHg) | 66.0 (7) | 71.0 (9)*** | 0.001 | 65.0 (17) | 70.0 (11)* | 0.028 |
| Heart rate (bpm) | 83.0 (15) | 103.0 (15)**** | 0.0001 | 85.0 (17) | 97.0 (16)**** | 0.0001 |
| P300 amplitude (µv) | 18.9 (10.3) | 17.3 (10.1) | 0.811 | 18.9 (7.8) | 18.0 (8.5) | 0.126 |
| P300 latency at Fz (ms) | 307.0 (30) | 305.0 (49) | 0.205 | 309.0 (33) | 309.0 (60) | 0.295 |
| P300 latency at Cz (ms) | 307.0 (26) | 296.0 (32)* | 0.024 | 307.0 (31) | 294.0 (43)* | 0.050 |
| P300 latency at Pz (ms) | 307.0 (23) | 297.0 (31)* | 0.033 | 307.0 (27) | 294.0 (37)# | 0.003 |
Data are presented as median (Interquartile range), ****p≤0.00001, ** p≤0.005, *** p≤0.001, *p≤0.05, #p≤0.01, Wilcoxon signed-rank test was employed for comparison of the variables.
It is evident from [Table 3] that there were significant changes in all cardiovascular parameters in post-exercise session in comparison to pre-exercise session in both phases of menstrual cycle. The amplitude and latencies of P300 ERP showed no significant difference at resting state (pre-exercise) during early follicular and mid-luteal phases. The amplitude of P300 ERP displayed no significant change in post-exercise session in comparison to pre-exercise session in both phases. However, P300 latency showed significant decrease in post-exercise session when recorded at Cz (P = 0.024, P = 0.05) as well as Pz position (P = 0.03, P = 0.003) in both phases of menstrual cycle. However, P300 latency recorded at Fz position displayed no significant change at post-exercise session in comparison to pre-exercise session during both phases of menstrual cycle.
MANCOVA analysis revealed that only amplitude of P300 ERP (P = 0.023) in post-exercise session during mid-luteal phase was affected by basal activity level significantly. However, amplitude and latencies of P300 ERP in other experimental conditions were not affected by activity level.
DISCUSSION
The present study intended to determine the effect of acute bout of moderate exercise on cognitive processing in young women during early proliferative and mid-luteal phase of menstrual cycle. The present study documented that only six participants were active and rest were insufficiently active. Following step-test exercise, one participant perceived exertion as extremely light and five of them perceived exertion as very light. The rest of them perceived the exertion as light to somewhat hard. It is conceivable that barring six participants, other participants are not so active at leisure time.
The cardiovascular parameters of the participants were recorded at rest and post-exercise. It was found that there was significant increase in both central and peripheral blood pressure and heart rate following exercise during both phases of menstrual cycle, which are well-established facts.
There is no consistent finding in the literature regarding changes in latency and amplitude of P300 ERP during menstrual cycle. It was reported earlier that ERPs inclusive of P300 elicited with auditory discrimination paradigm in women on the 1st day of menstrual cycle and approx. 14 days later revealed no change in amplitude or latency. Moreover, there was no difference noted in the response between the women who were on OCP and not on OCP. Therefore, the study concluded that menstrual cycle and use of oral contraceptives do not affect P300 and other ERP components.[17] Later on, a cross-sectional study was conducted to examine the changes in P300 component of visual ERPs and in BAEPs across the menstrual cycle in healthy women. It was documented that latency of P300 was longer during ovulatory phase.[18] Another study reported that amplitude of P300 ERP was significantly greater during menses than ovulatory phase. The study concluded that context updating mechanisms as indexed by P300 ERP are sensitive to cyclic hormonal fluctuations.[19] It is understandable that there is no agreement regarding changes in amplitude and latency of P300 ERP in different phases of menstrual cycle. The present study did not find any significant difference in amplitude and latency of P300 ERP recorded at resting state (pre-exercise) during early follicular and midluteal phase. The findings of various studies conducted on effects of menstrual cycle on cognitive functions have been fairly inconsistent. This could be explained based on the fact that oestrogen and progesterone have known to exert opposite effects on a variety of neurotransmitters in the brain. Both stimulatory and inhibitory effects of ovarian hormones on glutamatergic system have been reported.[9] It was documented that progesterone significantly enhanced GABAergic transmission and suppressed glutamatergic transmission.[20] On the other hand, it was documented that oestradiol enhanced glutamatergic neurotransmission.[21] Antagonistic effects on dopamine neurotransmission have also been noted.[9] Therefore, it might be construed that high progesterone level and low oestrogen peak during mid-luteal phase may cancel each other’s action which has been reflected in no significant difference in task performance.
Many research studies documented the beneficiary effect of acute bout of exercise on improving cognitive function of brain which involves response inhibition, cognitive flexibility, selective attention and working memory. It was reported earlier that there were differential influences of mild, moderate and high-intensity pedaling exercise on P300 ERP. The authors observed that amount of attentional resources to a given task was decreased with high-intensity pedaling exercise, but it was increased with moderate-intensity pedaling exercise. However, there was no change after low-intensity pedaling exercise. Hence, it was inferred that differences in exercise intensity influenced information processing in the CNS.[22] Another study conducted in India documented that latency of P300 was significantly decreased in sedentary individuals following acute moderate exercise.[15] It was further reported that acute bout of physical exercise causes reduction in P300 ERP latency and reaction times in both athlete and non-athlete groups.[23] It is to be reminded that most of the study participants were not active enough. The current study documented that significant decrease in P300 ERP latency in post-exercise session when recorded at Cz (P = 0.024, P = 0.05) as well as Pz position (P = 0.03, P = 0.003) in early proliferative and mid-luteal phases of the menstrual cycle. However, no significant change in P300 ERP latency was observed at Fz position at post-exercise session in both phases. It is known that P300 potential has subcomponents such as P3a and P3b. The P3a component has maximal amplitude over central/parietal region and is elicited by an infrequent stimulus in the absence of a task.[24] However, P3b potential is task relevant and its maximal amplitude is observed at parietal regions.[25,26] Latency of P3b reflects stimulus classification speed which is proportional to the time required to detect and evaluate a target stimulus. Therefore, any change in latency of P3b reflects underlying process of attention allocation and immediate memory.[27] In the present study, P300 latency has been reduced during post-exercise session which reflects enhanced attention allocation and faster information processing. However, the amplitude of P300 ERP displayed no significant change in post-exercise session in comparison to pre-exercise session in both phases. Interestingly, inferential statistical analysis revealed that only amplitude of P300 ERP (P = 0.023) in post-exercise session during mid-luteal phase was affected by activity level of the participants significantly. However, amplitude and latencies of P300 ERP in other experimental conditions were not affected by activity level. Taken together, it seems that the findings of the present study are more or less in concordance with the previous studies. Therefore, it can be inferred that exercise enhances release of monoamine system and several neurohormones, namely endorphins and BDNF, which act in a concerted fashion to bring about significant change in selective attention, response engagement, working memory and cognitive flexibility which are of paramount importance in dealing day-to-day situation in life.
CONCLUSION
The present study documented that an acute bout of moderate exercise caused significant decrease in latency of P300 ERP in the participants during both phases of menstrual cycle. Therefore, it can be stated that even acute bout of moderate exercise significantly enhances attention allocation, working memory in the participants; thereby, it enhances cognitive functioning of the individual.
Strengths, limitations and future directions
The strength of the study is that the same subject participated during both phases of the menstrual cycle. Hence, it is within subject comparison study. However, several limitations of the present study have to be considered to interpret the results. First of all, the sample size is less in the present study as it was a pilot one. Future studies can be conducted with more number of participants. Second, step test can be replaced with tread mill or bicycle ergometer based exercise stress tests which follow an automated fixed protocol. A randomised controlled trial in subjects with mild cognitive impairment (MCI) may be conducted in future to determine the beneficial effect of acute bout of moderate exercise on attention allocation and working memory. Third, the present study cannot find any significant difference of latency and amplitude of P300 ERP at basal level in two phases of menstrual cycle. However, few previous studies have reported change in amplitude and latency in different phases of menstrual cycle.[17-19] Overall, the findings of various similar studies conducted on effects of menstrual cycle on cognitive functions have been fairly inconsistent. The present study included only two phases of the menstrual cycle that is, early follicular (low level of both ovarian hormones) and mid-luteal phase. During mid-luteal phase, a higher progesterone peak and lower oestrogen peak occur. Therefore, if pre-ovulatory phase can be included in the study design, the effects of two ovarian hormones can be disentangled because pre-ovulatory phase is characterised by high level of oestrogen. Fourth, estimation of hormonal level in the participants can be done in the future studies to confirm the phase of menstrual cycle.
Acknowledgements
The authors acknowledge the contributions of the participants in the present study. The authors also acknowledge the helping hand provided by neurotechnician Mr. Mazhar Khan in optimising the procedure.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Ms. Rutuja Challawar was the recipient of ICMR Short Term Studentship program award for the year 2019.
Conflicts of interest
There are no conflicts of interest.
References
- Estrogen and cognitive functioning in women. Endocr Rev. 2003;24:133-51.
- [CrossRef] [PubMed] [Google Scholar]
- The role of hypothalamic-pituitary-gonadal hormones in the normal structure and functioning of the brain. Cell Mol Life Sci. 2005;62:257-70.
- [CrossRef] [PubMed] [Google Scholar]
- Emotional and cognitive functional imaging of estrogen and progesterone effects in the female human brain: A systematic review. Psychoneuroendocrinology. 2014;50:28-52.
- [CrossRef] [PubMed] [Google Scholar]
- Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci. 2015;9:37.
- [CrossRef] [PubMed] [Google Scholar]
- Estrogen effects on cognitive and synaptic health over the lifecourse. Physiol Rev. 2015;95:785-807.
- [CrossRef] [PubMed] [Google Scholar]
- Progesterone receptors: Form and function in brain. Front Neuroendocrinol. 2008;29:313-39.
- [CrossRef] [PubMed] [Google Scholar]
- Estrogen therapy selectively enhances prefrontal cognitive processes: A randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause. 2006;13:411-22.
- [CrossRef] [PubMed] [Google Scholar]
- Menstrual cycle influence on cognitive function and emotion processing-from a reproductive perspective. Front Neurosci. 2014;8:380.
- [CrossRef] [PubMed] [Google Scholar]
- The cycling brain: Menstrual cycle related fluctuations in Hippocampal and fronto-striatal activation and connectivity during cognitive tasks. Neuropsychopharmacology. 2019;44:1867-75.
- [CrossRef] [PubMed] [Google Scholar]
- Sex and the development of Alzheimer's disease. J Neurosci Res. 2017;95:671-80.
- [CrossRef] [PubMed] [Google Scholar]
- Biological markers of cognition in exercise: A mini review. Int J Clin Exp Physiol. 2019;6:78-81.
- [CrossRef] [Google Scholar]
- Be smart, exercise your heart: Exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58-65.
- [CrossRef] [PubMed] [Google Scholar]
- Failure to identify an acute exercise effect on executive function assessed by the Wisconsin Card sorting test. J Sport Health Sci. 2015;4:64-72.
- [CrossRef] [Google Scholar]
- A review of chronic and acute physical activity participation on neuroelectric measures of brain health and cognition during childhood. Prev Med. 2011;52:S21-8.
- [CrossRef] [PubMed] [Google Scholar]
- A simple method to assess exercise behaviour in the community. Can J Appl Sport Sci. 1985;10:141-6.
- [Google Scholar]
- Effect of acute moderate exercise on cognitive P300 in persons having sedentary lifestyles. Int J Appl Basic Med Res. 2012;2:67-9.
- [CrossRef] [PubMed] [Google Scholar]
- P300 and the menstrual cycle. Electroencephalogr Clin Neurophysiol. 1988;71:157-60.
- [CrossRef] [Google Scholar]
- Menstrual cycle synchronized changes in brain stem auditory evoked potentials and visual evoked potentials. Biol Psychiatry. 1999;45:1516-9.
- [CrossRef] [Google Scholar]
- The effect of the menstrual cycle on electrophysiological and behavioral measures of memory and mood. Psychophysiology. 2004;41:592-603.
- [CrossRef] [PubMed] [Google Scholar]
- Progesterone alters GABA and glutamate responsiveness: A possible mechanism for its anxiolytic action. Brain Res. 1987;400:353-9.
- [CrossRef] [Google Scholar]
- Estrogen enhances depolarization-induced glutamate release through activation of phosphatidylinositol 3-kinase and mitogen-activated protein kinase in cultured hippocampal neurons. Mol Endocrinol. 2003;17:831-44.
- [CrossRef] [PubMed] [Google Scholar]
- Differential influences of exercise intensity on information processing in the central nervous system. Eur J Appl Physiol. 2004;92:305-11.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of Moderate and high-intensity exercise on P300 latency and reaction time in athletes and non-athletes-an interim analysis. Biomedicine. 2016;36:60-5.
- [Google Scholar]
- Differences in spatio-temporal distribution of the visual P3b event-related potential between young men and women. Acta Neurobiol Exp (Wars). 2019;79:25-38.
- [CrossRef] [Google Scholar]
- Updating P300: An integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128-48.
- [CrossRef] [PubMed] [Google Scholar]
- P300 brain wave extraction from EEG signals: An unsupervised approach. Expert Syst Appl. 2017;74:1-10.
- [CrossRef] [Google Scholar]
- P300 as a clinical assay: Rationale, evaluation, and findings. Int J Psychophysiol. 2000;38:3-19.
- [CrossRef] [Google Scholar]






