Translate this page into:
Effect of Nyctanthes arbor-tristis on cardiovascular parameters and metabolic syndrome in fructose-induced hypertensive rats
*Corresponding author: Mahalaxmi Mohan, Department of Pharmacology, Mahatma Gandhi Vidyamandir’s Pharmacy College, Nashik, Maharashtra, India. mm_nasik@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Mohan M, Malode P, Pekhale D, Patodkar H. Effect of Nyctanthes arbor-tristis on cardiovascular parameters and metabolic syndrome in fructose-induced hypertensive rats. Indian J Physiol Pharmacol 2022;66:257-67.
Abstract
Objectives:
We investigated the effects of methanolic extract of Nyctanthes arbor-tristis (MNAT) 100, 200 and 400 mg/kg/day post-operative for 6 weeks on ECG, basal mean arterial blood pressure (MABP), heart rate, respiratory rate, vascular reactivity, antioxidant activities of enzyme superoxide dismutase (SOD) and catalase (CAT), levels of thiobarbituric acid reactive substances (TBARS), serum levels of leptin, adiponectin, glucose, triglycerides, cholesterol, uric acid, insulin, sodium and potassium in fructose-fed rats.
Materials and Methods:
A high-fructose-diet (fructose 10%, w/v) ad libitum for 6 weeks was used to induce hypertension in male Wistar rats (150–200 g). Sixty albino Wistar rats were randomly divided into a group of six, each group containing 10 animals. Group I was considered as normal control which received chow pellets and normal drinking water ad libitum for 6 weeks. Group II received fructose (10%) solution instead of normal drinking water for 6 weeks. Group III received fructose (10%) solution instead of drinking water ad libitum and MNAT at a dose of 100 mg/kg post-operative for 6 weeks. Group IV received fructose (10%) solution instead of drinking water ad libitum and MNAT at a dose of 200 mg/kg post-operative for 6 weeks. Group V received fructose (10%) solution instead of drinking water ad libitum and MNAT at a dose of 400 mg/kg post-operative for 6 weeks. Group VI received fructose (10%) solution instead of drinking water ad libitum and enalapril at a dose of 10 mg/kg post-operative for 6 weeks. Physiological parameters, ECG, heart rate, respiratory rate and blood pressure vascular reactivity to various drugs were measured and recorded by the invasive method. The antioxidant activities of enzyme SOD and CAT, levels of TBARS, along with serum levels of leptin, adiponectin, glucose, triglycerides, cholesterol, uric acid, insulin, sodium and potassium were measured. Cumulative concentration-response curve (CCRC) of Ang II and acetylcholine (Ach) was recorded.
Results:
MNAT treatment decreased MABP and altered vascular reactivity to various catecholamines. The activities of SOD and CAT enzymes exhibited a considerable increase and the levels of TBARS in the liver were reduced by MNAT treatment. MNAT has shown decrease in the plasma level of triglycerides, cholesterol, insulin and sodium while increase in plasma adiponectin and potassium levels. The CCRC of Ang II was shifted towards the right by MNAT treatment using an isolated strip of rat ascending colon. MNAT treatment increased the contractile characteristics of the rat ascending colon in the CCRC of ACh as compared to the fructose-treated group. MNAT treatment reduced fructose-induced tissue damage due to the consequence of metabolic syndrome (MetS). MNAT is rich in flavonoids and, therefore, has powerful antioxidant properties. The findings show that by battling oxidative stress caused by fructose (10%) and reducing Ang II activity, MNAT may be able to prevent the development of high blood pressure caused by fructose.
Conclusion:
MNAT has antihypertensive action and reverses MetS in the fructose-induced hypertensive rat model.
Keywords
Fructose
Metabolic syndrome
Hypertension
Oxidative stress
Insulin resistance
Hyperinsulinaemia
Hyperglycaemia
Dyslipidaemia
Nyctanthes arbor-tristis
INTRODUCTION
Nyctanthes arbor-tristis (NAT) Linn. (Oleaceae) is commonly known as Night jasmine, Parijata or Harshringar. It is used in the Ayurveda and Unani systems of medicine. Preliminary phytochemical investigation of NAT leaves showed the presence of flavonoids, phenolic compounds, tannins, saponins, alkaloids and steroids. The leaves of the plant possess different activities such as antioxidant, antibacterial, antifungal, anti-inflammatory, hepatoprotective and immunomodulatory.[1] According to a previous study, the methanolic extract of NAT leaves contains a variety of phytochemical substances with antioxidant activity that could be used to treat oxidative stress-related disorders.[2] Insulin resistance (IR), hypertension, hyperinsulinaemia, dyslipidaemia, oxidative stress and visceral obesity with a pro-inflammatory state are among the clinical and biochemical characteristics of metabolic syndrome (MetS). Several epidemiological studies have found a progressive link between dietary fructose consumption and the development of MetS. Fructose is a common sweetener found in soft drinks and other foods. In particular, a significant increase in obesity and the accompanying changes in MetS in the United States has been attributed to a 30% overall increase in fructose consumption.[3] Diet-induced hypertension (fructose-induced hypertension) is most often used method in rodents for induction of hypertension that is thought to be analogous to the human MetS, which is defined by the symptoms of dyslipidaemia, hyperglycaemia, IR, hyperinsulinaemia and hypertension.[4] Furthermore, high-fructose-fed rats have been demonstrated to have altered lipid metabolism as a result of hepatic stress caused by fructose metabolism.[5] Fructose and its metabolites may have a role in the generation of intracellular advanced glycation end-products (AGEs) and vascular dysfunction.[6] It has been suggested that the formation of reactive oxygen species (ROS) contributes to fructose-induced hypertension.[7] Flavonoid compounds found in a variety of plants have been demonstrated to have therapeutic benefits in cardiovascular disorders such as atherosclerosis, coronary artery disease and hypertension.[8] As NAT extracts contain flavonoids and are known for their antioxidant potential,[9] we hypothesised that NAT might have antihypertensive properties in fructose model. The goal of this study was to determine the effects of methanolic extract of Nyctanthes arbor-tristis (MNAT) and see if it might prevent hypertension and other abnormalities caused by a high-fructose diet in normal rats.
MATERIALS AND METHODS
Experimental animals
Sixty male albino Wistar rats (110–150 g) purchased from Mumbai Veterinary College (Mumbai, Maharashtra) were used in the current experimental study. The animals were kept under standard laboratory condition temperature 25 ± 1°C, relative humidity 45–55% and photoperiod (12 h dark/12 h light). The protocol of the study was approved by the Institutional Animal Ethical Committee (IAEC) (Approval No. MGV/PC/CPCSEA/XXXVII/01/2020/04). The date for IAEC was 24-10-2020.
Drugs and chemicals
Fructose, petroleum ether (60–80°C), methanol, sodium carbonate, sodium bicarbonate, hydrogen peroxide and gallic acid were obtained from Modern Sciences Pharmaceuticals, Nashik. Adrenaline (Adr), norepinephrine (NA), acetylcholine (ACh), angiotensin II (Ang II), phenylephrine (PE), urethane and 5-hydroxytryptamine (5-HT) were obtained from Sigma, Mumbai. MNAT was dissolved in distilled water and given orally according to the experimental protocol.
Preparation of the extract
Fresh leaves of NAT were purchased locally and authenticated by the Department of Pharmacognosy, MGV’s Pharmacy College, Nashik. Leaves were washed and dried in sunlight. The powder obtained (1 kg) was defatted using pet ether (60– 80°C) and extracted with methanol by hot extraction method using Soxhlet apparatus. The methanolic extract obtained was allowed for distillation to remove the excess quantity of methanol and to concentrate the product into a dry mass. The percentage yield value was found to be 12.89% w/w.[10]
Phytochemical analysis
The presence of various phytochemicals in MNAT was determined based on the standard qualitative test such as Dragendorff ’s test for alkaloid, FeCl3 for tannins, frothing test for saponin, Salkowski test for steroids, Shinoda test for flavonoids, Folin’s test for phenol, Molisch’s test for carbohydrates and Biuret test for proteins and amino acids.[10] Quantitative analysis was carried out for total flavonoid and phenolic content and in vitro antioxidant activity of MNAT was estimated using DPPH assay.[11]
Total flavonoid content
Flavonoids were determined using the aluminium chloride colorimetric technique. MNAT (0.5 ml of 1:20 g/ml) in methanol was combined with 11.5 ml of methanol, 0.1 ml of 10% aluminium chloride, 0.1 ml of IM potassium acetate and 2.8 ml of distilled water individually. It was kept at room temperature for 30 min. The reaction mixture’s absorbance was measured at 415 nm. The calibration curve was prepared using rutin solutions at concentrations 10–100 µg/ml in methanol.
Total phenol content
The total phenolic content of the MNAT extract was determined using spectrophotometric method (UV 2600, Shimadzu). The reaction mixture was prepared by mixing 0.5 ml of methanolic solution of MNAT and 2.5 ml of 10% Folin–Ciocalteu’s reagent. Blank was concomitantly prepared, containing 0.5 ml methanol and 2.5 ml of 10% Folin–Ciocalteu’s reagent. The samples were then incubated at room temperature for 45 min. The absorbance was recorded at 750 nm. Phenolic contents were measured using a standard curve obtained from various concentrations of gallic acid and expressed as microgram per milligram of gallic acid equivalents.
DPPH assay method
Free radical scavenging ability of MNAT extract was tested by DPPH radical scavenging assay. Various sample concentrations of the extract were divided into 5 µl, 10 µl, 20 µl, 40 µl and 80 µl test tubes. Each tube received 3 ml of 0.1 mM DPPH in ethanol and was incubated for 30 min the dark at room temperature. At 517 nm, the absorbance was measured (UV 2600, Shimadzu). The standard utilised was ascorbic acid. The percentage of inhibition was measured using the formula
Experimental protocol
A high-fructose diet (fructose 10%, w/v) ad libitum for 6 weeks was used to induce hypertension in male Wistar rats (150–200 g).[12] Sixty albino Wistar rats were randomly divided into a group of six, each group containing 10 animals. The doses were selected based on the previous literature and Smith schedule (1961).
Group I was considered as normal control which received chow pellets and normal drinking water ad libitum for 6 weeks
Group II received fructose (10%) solution instead of normal drinking water for 6 weeks
Group III received fructose (10%) solution instead of drinking water ad libitum and MNAT at a dose of 100 mg/kg post-operative for 6 weeks
Group IV received fructose (10%) solution instead of drinking water ad libitum and MNAT at a dose of 200 mg/kg post-operative for 6 weeks
Group V received fructose (10%) solution instead of drinking water ad libitum and MNAT at a dose of 400 mg/kg post-operative for 6 weeks
Group VI received fructose (10%) solution instead of drinking water ad libitum and Enalapril at a dose of 10 mg/kg post-operative for 6 weeks.
Physiochemical parameters
Food intake, water intake and body weight were monitored throughout the treatment. At the end of treatment schedule, average food intake, water intake, body weight and relative organ weight were reported for all the groups.
ECG recording, tracheostomy and measurement of blood pressure by invasive (direct) methods
After the treatment schedule was completed, a subset of five rats from each group was used for ECG recording, tracheostomy-based respiration rate measurement and invasive blood pressure measurement, as described by Parasuraman and Raveendran (2012). Urethane (1200 mg/kg, i.p.) was used to anaesthetise the rats.
Tracheostomy was performed to monitor the respiration rate. For blood pressure measurement, the left common carotid artery was cannulated using polyethylene tubing which was prefilled with heparinised saline (100 IU/ml) to prevent clotting. The cannula was connected to a pressure transducer by a direct method onto a chart data system (PowerLab4/35; AD Instruments, Sydney, Australia). The left femoral vein was cannulated for the administration of various drugs. After the stabilisation period of 30 min, basal mean arterial blood pressure (MABP), heart rate, respiratory rate and vascular reactivity to Adr (1 µg/kg), NA (1 µg/kg), PE (1 µg/kg), Ang II (25 ng/kg) and 5-HT (1 µg/kg) were recorded.[13]
Biochemical analysis and estimation of antioxidants
At the end of the treatment schedule, another subset of five rats was anaesthetised, and blood was collected by cardiac puncture using a 25G needle with a 5 ml syringe.[14] Plasma levels of leptin and adiponectin were determined by enzyme-linked immunosorbent assay (ELISA). Plasma glucose (GOD-POD method), triglycerides (GPO-PAP endpoint assay), cholesterol (CHOD-PAP enzymatic endpoint assay), uric acid (uricasePOD endpoint assay), insulin (ELISA) and sodium and potassium (ion-selective electrodes method) were determined from Immuno Chem Technology Pvt. Ltd., Nashik.
The animals were sacrificed and the liver was immediately removed and cleaned in ice-cold saline. Liver tissue was homogenised. The activity of enzymes such as superoxide dismutase (SOD), catalase (CAT) and thiobarbituric acid reactive substance (TBARS) was measured in the supernatants.[15]
In vitro studies and histopathological study
After completion of blood sampling, individual groups of rats were sacrificed. Rat ascending colon was isolated and used for the cumulative concentration-response curve (CCRC) for Ang II[16] and Ach.[17]
From individual groups, kidneys, liver, aorta and heart were isolated, weighed and fixed in 10% formalin. Haematoxylin and eosin (H and E staining) were used to stain 5 µm slices of samples, which were then examined under ×40 using light microscope.
Statistics
For each group, the mean SEM values were determined. For statistical analysis, one-way ANOVA was performed, followed by Dunnett’s multiple comparison tests. Statistical significance was defined as P < 0.05.
RESULTS
Phytochemical analysis
The total flavonoid content of the MNAT was found to be 38 µg rutin Equiv/mg of extract. The total phenolic content of MNAT was found to be 42.91 µg gallic acid Equiv/mg of extract. Free radical scavenging activity of MNAT was evaluated using in vitro DPPH assay, the 50% inhibitory effect of the extract was calculated from the curve and was found to be 1.8 µg/ml. Appropriate doses of the extract were made in distilled water. The phytoconstituents present in the crude extract were flavonoids, alkaloids, tannins, steroids and saponins.
Physiological parameters, basal MABP, heart rate and respiratory rate
After the treatment schedule, fructose-fed rats showed a significant increase in fluid intake, body weight, relative organ weight, basal MABP, heart rate and respiratory rate, while the food intake was decreased significantly as compared to the control. After giving treatment with MNAT (100, 200 and 400 mg/kg, post-operative, for 6 weeks), there was a significant decrease in the fluid intake, body weight, relative organ weight, basal MABP, heart rate and respiratory rate while the food intake was increased significantly as compared to the fructose-fed rats [Table 1].
| Parameters | Group (mg/kg) | |||||
|---|---|---|---|---|---|---|
| Control | Fructose (10%) | F+MNAT (100) | F+MNAT (200) | F+MNAT (400) | F+Enal (10) | |
| Food intake (g/day/animal) | 17.68±0.66 | 14.3±0.94* | 15.78±0.16 | 16.6±0.99# | 18.16±0.23# | 17.77±0.29# |
| Fluid intake (ml/day/animal) | 28.66±0.59 | 72.57±3.06* | 63.93±3.71 | 59.89±3.03# | 56.57±2.66# | 61.38±5.39# |
| % gain in body weight | 72.83±5.64 | 90.8±4.45* | 74.5±5.05# | 65.1±6.23# | 68.1±1.14# | 68.4±2.70# |
| Liver weight (g/100 g BW) | 3.474±0.14 | 4.464±0.20* | 3.245±0.02# | 3.306±0.09# | 3.163±0.13# | 2.850±0.11# |
| Heart weight (g/100 g BW) | 0.389±0.02 | 0.449±0.02* | 0.371±0.004# | 0.362±0.008# | 0.344±0.02# | 0.304±0.01# |
| Left kidney weight (g/100 g BW) | 0.391±0.02 | 0.434±0.02* | 0.361±0.004# | 0.360±0.008# | 0.354±0.01# | 0.308±0.009# |
| Right kidney weight (g/100 g BW) | 0.395±0.02 | 0.460±0.03* | 0.384±0.01# | 0.376±0.01# | 0.337±0.02# | 0.311±0.01# |
| Basal MABP (mm Hg) | 94.2±1.8 | 128.6±5.6* | 82.6±1.63# | 74.8±2.74# | 72±2.05# | 71.4±2.24# |
| Heart rate (beats/min) | 295±3.64 | 355.6±7.82* | 340.8±4.94 | 281±3.0# | 282±10.8# | 320.6±4.6# |
| Respiratory rate (breaths/min) | 83.2±0.93 | 107±3.91* | 85±2.63# | 84.75±0.76# | 84.75±1.29# | 80.5±0.52# |
All values are expressed as mean±SEM, n: 5. All data are subjected to one-way ANOVA followed by Dunnett’s test *P<0.05 when compared to control and #P<0.05 when compared to the fructose-treated group. The gain in body weight was noted after 6 weeks. MNAT: Methanolic extract of Nyctanthes arbor-tristis, Enal: Enalapril, F: Fructose (10%)
Effect of MNAT on ECG parameters
Fructose-fed rats showed a significant decrease in P wave duration, RR interval, ST height and T amplitude and showed significant elevation in QRS interval, QT interval and R amplitude. The MNAT-treated group showed improvement in ECG parameters. MNAT (400) treatment rats showed a significant increase in P wave duration, RR interval, ST height and T amplitude and showed a significant decrease in QRS interval, QT interval and R amplitude which were moreover similar to control rats [Table 2].
| Parameters | Group (mg/kg) | |||||
|---|---|---|---|---|---|---|
| Control | Fructose (10%) | F+MNAT (100) | F+MNAT (200) | F+MNAT (400) | F+Enal (10) | |
| P interval (sec) | 0.021106±0.000939 | 0.01929±0.000189* | 0.020264±0.000633 | 0.022004±0.00983 | 0.024462±0.000745# | 0.023574±0.002226# |
| QRS interval (sec) | 0.01443±0.002307 | 0.01651±0.000002* | 0.01522±0.000147# | 0.0145±0.000289 | 0.01268±0.000540# | 0.01398±0.000259# |
| QT interval (sec) | 0.05602±0.002039 | 0.07505±0.003429* | 0.05543±0.001835# | 0.04756±0.001838# | 0.04308±0.002736# | 0.05048±0.000676# |
| RR interval (sec) | 0.2603±0.002426 | 0.1343±0.000359* | 0.2363±0.001287# | 0.2752±0.000650# | 0.3445±0.009088# | 0.2769±0.003955# |
| R amplitude (mv) | 0.5055±0.001959 | 0.5659±0.002949* | 0.4811±0.00393# | 0.4887±0.003321# | 0.3755±0.002829# | 0.4832±0.006533# |
| ST height (mv) | 0.1865±0.000317 | 0.07697±0.01412* | 0.09159±0.002192 | 0.1342±0.003526# | 0.1836±0.001183# | 0.187±0.002563# |
| T amplitude (mv) | 0.2352±0.002252 | 0.15±0.002945* | 0.1317±0.002432# | 0.1851±0.002917# | 0.2185±0.000549# | 0.2058±0.003658# |
All values are expressed as mean±SEM, n: 5. All data are subjected to one-way ANOVA followed by Dunnett’s test *P<0.05 when compared to control and #P<0.05 when compared to the fructose-treated group. MNAT: Methanolic extract of Nyctanthes arbor-tristis, Enal: Enalapril, F: Fructose (10%)
Effect of MNAT vascular reactivity
The pressor response to Adr, noradrenaline, PE, Ang II and 5-HT was significantly increased. In MNAT-treated rats (100, 200 and 400 mg/kg, post-operative, for 6 weeks), the vasoconstrictor response decreased significantly to Adr, noradrenaline, PE, Ang II and 5-HT. The response of the MNAT (400 mg/kg)-treated group to vasoconstrictor stimuli was equivalent to enalapril-treated group [Figure 1].

- Effect of MNAT (100, 200 and 400 mg/kg, p.o., for 6 weeks) on vascular reactivity changes in arterial blood pressure to various drugs: Adrenaline (Adr-1 µg/kg), Nor adrenaline (NA 1 µg/kg), Phenylephrine (PE-1 µg/kg), Angiotensin II (Ang II-25 ng/kg), 5- hydroxytryptamine (5-HT-1 µg/kg). All values are expressed as mean±SEM, n = 5. All data are subjected to One Way ANOVA followed by Dunnett’s test. *P < 0.05 when compared to control and #P < 0.05 when compared to fructose-treated group. Vertical line represents SEM. F: fructose (10 %), MNAT: Methanolic Extract of Nyctanthes arbor-tristis, Enal: Enalapril
Biochemical parameters
Significant increase in the plasma leptin, glucose, triglycerides, cholesterol, uric acid, insulin and sodium level and a significant decrease in plasma adiponectin and potassium levels were observed in rats treated with fructose (10%) for 6 weeks as compared to the control group. MNAT (100, 200 and 400 mg/kg) and Enal (10 mg/kg) have shown a decrease in the level of plasma leptin, adiponectin, glucose, triglycerides, cholesterol, uric acid, insulin, sodium and potassium when compared with fructose (10%)-fed animals [Table 3].
| Parameters | Group (mg/kg) | |||||
|---|---|---|---|---|---|---|
| Control | Fructose (10%) | F+MNAT (100) | F+MNAT (200) | F+MNAT (400) | F+Enal (10) | |
| Leptin (ng/ml) | 2.52±0.37 | 5.45±1.13* | 4.31±0.19 | 3.13±0.1# | 2.78±0.67# | 2.98±0.28# |
| Adiponectin (ng/ml) | 3.97±0.19 | 1.25±0.21* | 1.75±0.12 | 2.34±0.19# | 2.93±0.1# | 3.38±0.47# |
| Glucose (mg/dl) | 119.4±6.42 | 248.8±8.86* | 219.5±9.57 | 183.3±14.33# | 166±13.05# | 140.6±14.26# |
| Triglycerides (mg/dl) | 51.65±6.95 | 155±19.75* | 93.12±1.02# | 76.77±8.63# | 40.03±1.19# | 106.9±1# |
| Cholesterol (mg/dl) | 57.30±1.23 | 69.71±1.18* | 51.62±2.00# | 44.93±5.98# | 42.72±1.58# | 56.62±3.91# |
| Uric acid (mg/dl) | 3.65±0.43 | 6.55±1.68* | 4.05±0.69 | 3.13±0.17# | 2.33±0.89# | 3.28±0.2# |
| Insulin (ng/dl) | 2.58±0.2 | 5.66±0.26* | 3.83±0.34# | 3.28±0.58# | 2.75±0.23# | 3.14±0.57# |
| Insulin resistance index (HOMA) | 0.809±0.105 | 3.472±0.135* | 2.079±0.219# | 1.683±0.127# | 1.119±0.094# | 0.647±0.35# |
| Sodium (mmol/dl) | 145.9±0.31 | 166.9±3.85* | 138.5±3.06# | 129.6±3.56# | 127.6±5.96# | 126.8±0.56# |
| Potassium (mmol/dl) | 6.82±1.55 | 3.5±0.23* | 4.08±0.26 | 5.26±0.28 | 5.95±0.14# | 6.09±0.38# |
All values are expressed as mean±SEM, n: 5. All data are subjected to one-way ANOVA followed by Dunnett’s test *P<0.05 when compared to control and #P<0.05 when compared to the fructose-treated group. The gain in body weight was noted after 6 weeks. MNAT: Methanolic extract of Nyctanthes arbor-tristis,Enal: Enalapril, F: Fructose (10%)
Antioxidants parameters
The levels of SOD and CAT enzymes were significantly decreased and those of TBARS were significantly increased in liver tissue of fructose (10%)-fed rats when compared to control rats. The levels of SOD and CAT enzymes were significantly increased and TBARS were significantly decreased in liver tissue of MNAT (400 mg/kg) when compared to fructose (10%)-fed rats [Table 4].
| Parameters | Group (mg/kg) | |||||
|---|---|---|---|---|---|---|
| Control | Fructose (10%) | F+MNAT (100) | F+MNAT (200) | F+MNAT (400) | F+Enal (10) | |
| SOD (U/mg of tissue) | 0.48±0.052 | 0.12±0.032* | 0.23±0.046 | 0.34±0.012# | 0.37±0.076# | 0.39±0.030# |
| Catalase (U/mg of tissue) | 27.40±2.65 | 20.84±2.44* | 23.87±0.36 | 25.52±0.88 | 26.63±1.44# | 25.21±0.29 |
| TBARS (nmoles/mg of tissue) | 2.91±1.02 | 7.44±0.84* | 3.96±1.06 | 4.51±1.34 | 3.23±0.32# | 2.68±1.61# |
All values are expressed as mean±SEM, n=5. All data are subjected to one-way ANOVA followed by Dunnett’s test *P<0.05 when compared to control and #P<0.05 when compared to fructose-treated group. MNAT: Methanolic extract of Nyctanthes arbor-tristis, Enal: Enalapril, F: Fructose (10%), SOD: Superoxide dismutase, TBARS: Thiobarbituric acid reactive substance
In vitro study
When compared to the CCRC of fructose-fed rats, treatment of MNAT (100, 200 and 400 mg/kg/day, post-operative) for 6 weeks in fructose-fed rats significantly altered CCRC of Ang II to the right, with suppression of maxima [Figure 2]. For isolated ascending colon, administration of MNAT (400 mg/kg/day, post-operative) in fructose (10%)-fed rats for 6 weeks substantially enhanced percentage responsiveness of ACh compared to fructose (10%)-fed rats [Figure 3].
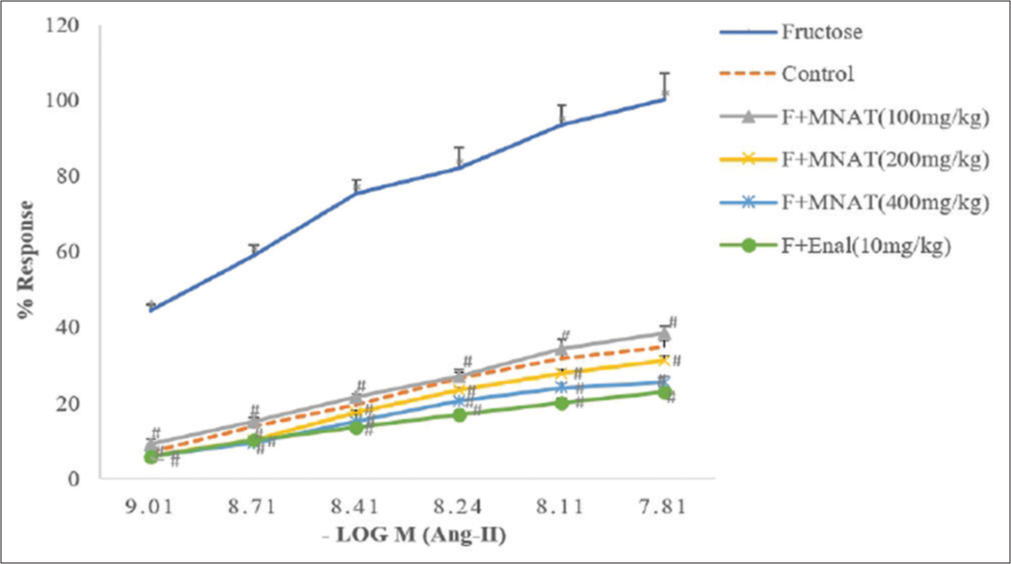
- Effect of MNAT on the cumulative concentration-response curve (CCRC) of angiotensin II on isolated rat ascending colon in rats. All values are expressed as mean ± SEM, n = 5. All data are subjected to One Way ANOVA followed by Dunnett’s test. *P < 0.05 when compared to control and #P < 0.05 when compared to the fructose-fed group. MNAT-Methanolic extract of Nyctanthes arbor-tristis, F- Fructose (10%), Enal-Enalapril
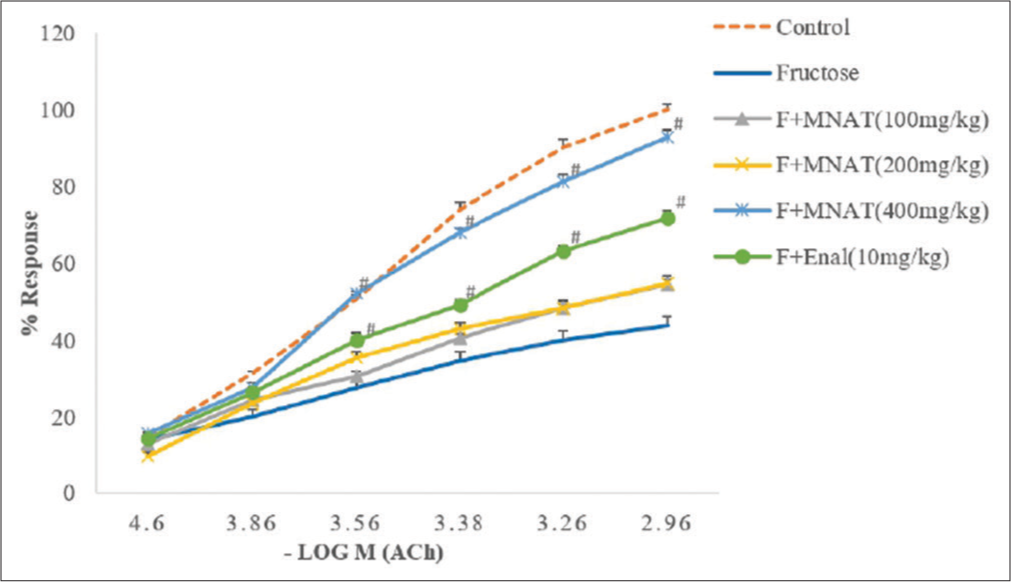
- Effect of MNAT on the CCRC of Ach on isolated rat ascending colon in rats. All values are expressed as mean ± SEM, n = 5. All data are subjected to One Way ANOVA followed by Dunnett’s test. *P < 0.05 when compared to control and P < 0.05 when compared to the fructose-fed group. MNAT-Methanolic extract of Nyctanthes arbor-tristis, F- Fructose (10%), Enal-Enalapril
Histopathological examination
In fructose-fed rats, liver showed the presence of macrovesicular steatosis, fat accumulation and congestion of blood sinusoids around the central vein, aorta showed increased thickness of tunica media, heart showed vacuolation of cardiomyocytes and mild hyaline degeneration and kidney showed renal hypertrophy compared to control group. MNAT (100, 200 and 400 mg/kg/day, post-operative)-treated rats have reversed the histological disturbances in liver, aorta, heart and kidney caused by fructose [Figures 4-8].

- Kidney histopathology (a) Kidney of the control group showed the normal histological picture, normal glomerulus (arrow). (b) Kidney of Fructose (10%) group showed cloudy swelling in renal tubules (asterisks), sclerotic glomerulus (arrow). (c and d) Kidney of MNAT (100 and 200 mg/kg) treated showed mild cloudy swelling in renal tubules (asterisks), normal glomerulus (arrow). (e and f) Kidney of MNAT (400 mg/kg) and Enal (10 mg/kg) group showed normal histological picture, normal glomerulus (arrow). (H&E, × 40). MNAT-Methanolic extract of Nyctanthes arbor-tristis, F- Fructose (10%), Enal-Enalapril

- Liver histopathology (a) Liver of control rats showed normal hepatic lobules and hepatocytes with normal architecture. (b) The liver of the Fructose (10%) group showed the presence of macrovesicular steatosis (Black arrows), fat accumulation (dotted arrow) and congestion of blood sinusoids around the central vein. (c) Liver of MNAT (100 mg/kg) treated showed mild macrovesicular steatosis (Black arrows), fat accumulation (dotted arrow) and congestion of blood sinusoids around the central vein. (d) Liver of MNAT (200 mg/kg) treated showed mild macrovesicular steatosis (Black arrows); fat accumulation (dotted arrow). (e and f) Liver of MNAT (400 mg/kg) and Enal (10 mg/kg) group showed normal hepatic lobules and hepatocytes with normal architecture. (H&E, × 40) MNAT-Methanolic extract of Nyctanthes arbor-tristis, F- Fructose (10%), Enal-Enalapril

- Aorta histopathology (a) Aorta of control rats (b) Aorta of Fructose (10%) group showed increased thickness of tunica media (Blackline) (c and d) Aorta of MNAT (100 and 200 mg/kg) treated showed the mildly decreased thickness of tunica media (Blackline) (e) Aorta of MNAT (400 mg/kg) treated showed a normal layer of tunica media (Blackline) (d) Aorta of Enal (10 mg/kg) showed the decreased thickness of tunica media (Blackline), (c-f) were compared only with fructose 10% treated group). (H&E, × 40). MNAT-Methanolic extract of Nyctanthes arbor-tristis, F-Fructose (10%), Enal – Enalapril.

- Heart histopathology (a) Heart of the control group shows a normal histological picture. (b) Heart of fructose 10% group shows vacuolation of cardiomyocytes (arrows) and mild hyaline degeneration (arrowhead). (c-f) Heart of MNAT (100,200 and 400 mg/kg) and Enal 10mg/kg treated showed mild hyaline degeneration (arrow head). (H&E, × 40). MNAT-Methanolic extract of Nyctanthes arbor-tristis, F Fructose (10%), Enal-Enalapril

- Representative gross images of kidneys (a) Kidney of the control group. (b) Kidney of Fructose (10%) treated group showing hypertrophy. (c-f) Kidney of MNAT (100, 200, and 400 mg/kg) and Enal (10 mg/kg) treated groups. MNAT-Methanolic extract of Nyctanthes arbor-tristis, F Fructose (10%), Enal-Enalapril
DISCUSSION
The findings on fructose-fed rats generally show symptoms such as IR, hyperinsulinaemia, hyperglycaemia and hypertriglyceridaemia which lead to hypertension.[7,18] In our study, the efficacy of MNAT was evaluated in high-fructose-fed albino Wistar rats. Overall, the data suggest that MNAT 400 mg has an efficacy comparable to enalapril.
The control group showed a standard ECG. Fructose (10%)-fed rats significant decrease in the P wave duration indicating enlargement of the atria which is due to the decreased ionotropic effect and RR interval with a significant rise in the heart rate. According to earlier research, both MetS and diabetic patients showed significant change in QRS complex which is due to deterioration of depolarisation sequence and similar modifications have been discovered in the present study with elevation in ST height, heart rate and other ECG components.[19] Improvement in ECG pattern was found in MNAT (100, 200 and 400 mg/kg)-treated fructose (10%) hypertensive rats.
It has been proven that a high-fructose diet causes hypertension through sympathetic over-activation, increased salt absorption and endothelial dysfunction.[20] The preventive impact of oestrogen and the potentiating role of testosterone are suggested by gender variations in fructose-induced hypertension development.[7] As a result, we used male rats in our present research work.
In the present study, fructose (10%)-treated rats showed a significant increase in basal MABP and respiratory rate and exaggerated vasopressor response to drugs such as Adr, noradrenaline, PE, Ang II and 5- HT. MNAT (100, 200 and 400 mg/kg) was able to prevent hypertension due to a fructose-rich diet. MNAT (100, 200 and 400 mg/kg) maintained normal basal MABP, and respiratory rate and also was able to prevent the exaggerated responses to the sympathetic agonist. This may be due to the presence of phytochemical constituents such as polyphenols and flavonoids which show antioxidant activity. Enalapril exhibited tremendous recovery of basal MABP and respiratory rate and prevent the exaggerated responses to the sympathetic agonist.
High-fructose diet causes decrease in leptin sensitivity and an elevation in body fat mass, which leads to increased leptin synthesis by fat tissues. According to the previous studies, the level of adiponectin a fat-derived hormone that plays crucial role in the development of obesity and cardiovascular disease is low in serum, a predictor of MetS.[21] In the present study, fructose (10%)-treated rats showed a significant increase in leptin levels and body weight while the food intake and adiponectin were significantly decreased. MNAT (100, 200 and 400 mg/kg) was able to the lower leptin levels while raising adiponectin levels.
Fructose raises free fatty acid (FFAs) levels, which contributes to IR and inhibition of pancreatic insulin production through poor insulin signalling. IR causes the body to produce more insulin. In fructose-fed rats, hyperglycaemia is related to hyperinsulinaemia. Increased glucose levels in fructose (10%)-fed rats may be attributed to a gluconeogenic condition in the liver and inadequate insulin stimulation for glucose oxidation in the liver, adipose tissue and muscle, according to earlier studies on glucose uptake.[22] The insulin and glucose levels in MNAT (100, 200 and 400 mg/kg)-treated rats were significantly lower. Yadav et al. demonstrated that in fructose-fed rats, IR is manifested by elevated plasma insulin, triglycerides, FFAs and HOMA. The degree of IR (HOMA-IR) in fructose-fed rats was found to be increased after the 6th week.[23] HOMA-IR was found to be reduced by administering MNAT (100, 200 and 400 mg/kg).
Fructose is more lipogenic than other carbohydrates or sugars and hence causes an elevation in triglycerides and cholesterol.[24] The rise in lipid and protein oxidative products indicates NADPH oxidase activation causing oxidative stress.[25]
In our present study, fructose (10%)-fed rats showed elevation in cholesterol and triglycerides. MNAT (100, 200 and 400 mg/kg) treatment was found to be effective to reduce the level of triglycerides and cholesterol.
In fructose-fed rats, uric acid levels rise, resulting in hyperuricaemia, which may play a role in MetS pathogenesis.[20] Fructose increases the level of sodium while depleting the level of potassium.[26] In our study, after the treatment with MNAT (100, 200 and 400 mg/kg), sodium levels and uric acid levels were found to be decreased while potassium levels increased.
Consumption of fructose enhances the generation of ROS and the reduction of antioxidant defence systems, resulting in increased blood lipid peroxidation susceptibility.[27] SOD and CAT activities were found to be decreased in high-fructose-fed insulin-resistant rats in the previous investigations.[28] In comparison to control rats, fructose (10%)-fed animals had greater amounts of TBARS. The results of SOD and CAT activity in our study demonstrated that MNAT (100, 200 and 400 mg/kg) treatment increased scavenging activity and depleted levels of TBARS.
Consumption of fructose may result in the build-up of AGE in smooth muscle cells, leading to a change in the contractile activity of the intestinal smooth muscles.[29] It has been found that fructose diet promotes sympathetic activity while decreasing parasympathetic activity.[30] The presence of a link between the endothelin and renin-Ang systems that could influence the development of fructose-induced hypertension is well established.[31] MNAT, which is rich in flavonoids, reduces fructose-induced hypertension in rats by inhibiting the production of ET-1 and Ang II. MNAT (100, 200 and 400 mg/kg) shifts the CCRC of Ang II to the right, indicating an inhibitory action on Ang II receptors. In the CCRC of Ach, the contractile properties of rat ascending colon are increased by MNAT (100, 200 and 400 mg/kg) treatment as compared to fructose-treated group. This indicates an improvement in parasympathetic activity which is decreased in fructose hypertensive rats. Long-term fructose consumption increases oxidative damage.[32] High-fructose administration resulted in disruption of normal histology of kidney, aorta and heart of rats.[33] Endotoxemia and increased release of inflammatory cytokines were associated with high-fructose diets, which can contribute to IR and fatty liver.[34] MNAT has potent antioxidant properties, which may reduce oxidative stress, and suppresses free radical formation. In fructose hypertensive rats, administration of MNAT (100, 200 and 400 mg/kg) reduced fat deposition in the liver, reduced aortic wall thickening and prevented glomerulosclerosis and cardiomyocyte vacuolation. In our study, MNAT (100, 200 and 400 mg/kg) has shown a protective effect on histology of kidney, liver, aorta and heart as compared to fructose (10%)-treated rats. In fructose (10%)-fed rats, glomerular hypertension, renal hypertrophy and cortical vasoconstriction are all indicators of renal dysfunction.[35] Renal hypertrophy was seen in fructose-fed rats compared to control rats and it was reversed by MNAT (100, 200 and 400 mg/kg) and enalapril administration.
CONCLUSION
As a result of our findings, MNAT has the potential to be employed as an adjuvant treatment to prevent and/or treat chronic disorders characterised by IR, hyperinsulinaemia, hyperglycaemia, hypertriglyceridaemia, exacerbated antioxidant status and hypertension caused by fructose.
The limitation of the study is that the work has been carried out with methanolic extract, the active principle responsible for activity needs to be tapped. Drug development including standardisation, stability studies and quality assurance, and clinical research (safety and efficacy) of the formulation can be worked on later as per the standard guidelines in the future.
Acknowledgments
The authors acknowledge AICTE MODROB for support of this work and thank Principal Dr. R. S. Bhambar for providing necessary laboratory facilities.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
AICTE-MODROB Grant.
References
- Traditional medicinal uses, phytochemical profile and pharmacological activities of Nyctanthes arbortris. Res J Life Sci Bioinform Pharm Chem Sci. 2019;5:1003-23.
- [Google Scholar]
- Comparative evaluation of antioxidant activity of aqueous and methanolic extract of Nyctanthes arbor-tristis Linn. by using DPPH assay. Int J Res Eng Sci Manag. 2020;3:68-70.
- [Google Scholar]
- Hypothesis: Fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. J Nat Clin Pract Nephrol. 2005;1:80-6.
- [CrossRef] [PubMed] [Google Scholar]
- Induction of hypertension by various animal models. Int J Pharm Biol Sci. 2011;1:335-40.
- [Google Scholar]
- High dietary fructose induces a hepatic stress response resulting in cholesterol and lipid dysregulation. Endocrinology. 2004;145:548-55.
- [CrossRef] [PubMed] [Google Scholar]
- Formation of fructose-mediated advanced glycation end products and their roles in metabolic and inflammatory diseases. Adv Nutr. 2017;8:54-62.
- [CrossRef] [PubMed] [Google Scholar]
- The fructose-fed rat: A review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol Cell Biochem. 2009;332:145-59.
- [CrossRef] [PubMed] [Google Scholar]
- Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen elderly study. Lancet. 1993;342:1007-11.
- [CrossRef] [PubMed] [Google Scholar]
- Antioxidant and anti-inflammatory activities of Nyctanthes arbor-tristis extracts. J Adv Bot Zool. 2016;4:1-5.
- [Google Scholar]
- Preliminary phytochemical analysis of methanolic and aqueous extract of medicinal plant-Nyctanthes arbor-tristis Linn. World J Pharm Pharm Sci. 2016;5:1393-401.
- [Google Scholar]
- Evaluation of antioxidant activity of flavonoid and phenolic contents of Luffa echinata Roxb. Fruits and Nyctanthus arbor-tristis leaves. Int J Phytopharm. 2011;1:8-14.
- [CrossRef] [Google Scholar]
- Fructose consumption enhances glucocorticoid action in rat visceral adipose tissue. J Nutr Biochem. 2013;24:1166-72.
- [CrossRef] [PubMed] [Google Scholar]
- Measurement of invasive blood pressure in rats. J Pharmacol Pharmacother. 2012;3:172-7.
- [Google Scholar]
- Blood sample collection in small laboratory animals. J Pharmacol Pharmacother. 2010;1:87-93.
- [CrossRef] [PubMed] [Google Scholar]
- Protective effect of Solanum torvum on monosodium glutamate-induced hepatotoxicity and nephrotoxicity in rats. Indian J Nat Prod Resour. 2019;10:31-42.
- [Google Scholar]
- A sensitive method for the assay of angiotensin. Br J Pharmacol Chemother. 1964;23:351-9.
- [CrossRef] [PubMed] [Google Scholar]
- Depressive effect of epinephrine mediated via adrenergic beta-receptor in isolated rat colon and duodenum. Jpn J Pharmacol. 1977;27:341-9.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of the aqueous and methylene chloride extracts of Bidens pilosa leaf on fructose-hypertensive rats. J Ethnopharmacol. 2001;76:215-21.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of diosmin and crocin on metabolic syndrome-associated cardio-vascular complications in rats. Naunyn Schmiedebergs Arch Pharmacol. 2019;392:1523-36.
- [CrossRef] [PubMed] [Google Scholar]
- The mechanisms underlying fructose-induced hypertension: A review. J Hypertens. 2015;33:912-20.
- [CrossRef] [PubMed] [Google Scholar]
- Leptin, adiponectin and insulin as regulators for energy metabolism in a rat model of metabolic syndrome. Sains Malays. 2019;48:2701-7.
- [CrossRef] [Google Scholar]
- Oreocnide integrifolia (Gaud.) Miq leaf water extract improves metabolic alterations in high fructose fed insulin resistant and hypertensive rats. Eur J Integr Med. 2010;2:79-87.
- [CrossRef] [Google Scholar]
- Epigenomic derangement of hepatic glucose metabolism by feeding of high fructose diet and its prevention by rosiglitazone in rats. Dig Liver Dis. 2009;41:500-8.
- [CrossRef] [PubMed] [Google Scholar]
- Fructose, insulin resistance, and metabolic dyslipidemia. Nutr Meta (Lond). 2005;2:5.
- [CrossRef] [PubMed] [Google Scholar]
- High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939-45.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of genistein, a soy isoflavone, on whole body insulin sensitivity and renal damage induced by a high-fructose diet. Ren Fail. 2008;30:645-54.
- [CrossRef] [PubMed] [Google Scholar]
- Fructose-enriched diet modifies antioxidant status and lipid metabolism in spontaneously hypertensive rats. Nutrition. 2006;22:758-66.
- [CrossRef] [PubMed] [Google Scholar]
- Fructose-fed rat hearts are protected against ischemia-reperfusion injury. Exp Biol Med (Maywood). 2006;231:456-62.
- [CrossRef] [PubMed] [Google Scholar]
- Metabolic disorders induced by fructosedrinking water affect angiotensin II-mediated intestinal contractility in male Wistar rats. Folia Med (Plovdiv). 2020;62:802-11.
- [CrossRef] [PubMed] [Google Scholar]
- The role of the baroreflex and parasympathetic nervous system in fructose-induced cardiac and metabolic alterations. Sci Rep. 2018;8:10970.
- [CrossRef] [PubMed] [Google Scholar]
- Endothelin-1 modulates angiotensin II in the development of hypertension in fructosefed rats. Mol Cell Biochem. 2009;325:89-97.
- [CrossRef] [PubMed] [Google Scholar]
- Spirulina maxima and its effect on antioxidant activity in fructose induced oxidative stress with histopathological observations. Eur Pharm J. 2015;62:13-9.
- [CrossRef] [Google Scholar]
- Effect of high fructose administration on histopathology of kidney, heart and aorta of rats. J Adv Vet Anim Res. 2017;4:71-9.
- [CrossRef] [Google Scholar]
- Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology. 2009;50:1094-104.
- [CrossRef] [PubMed] [Google Scholar]
- Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am J Physiol Renal Physiol. 2007;292:423-9.
- [CrossRef] [PubMed] [Google Scholar]






