Translate this page into:
In silico profiling of Cynodon dactylon L. Bioactives: Targeting Alzheimer’s pathways through network pharmacology, molecular docking and ADMET analysis
*Corresponding author: Laxmi Pattanashetti, Department of Pharmacology, KLE College of Pharmacy Hubli, (A constituent unit of KAHER, Belagavi) Karnataka, India. pattanashetti.laxmi67@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Pattanashetti L, Patil VS, Patil BM. In silico profiling of Cynodon dactylon L. Bioactives: Targeting Alzheimer’s pathways through network pharmacology, molecular docking and ADMET analysis. Indian J Physiol Pharmacol. 2025;69:63-76. doi: 10.25259/IJPP_617_2023
Abstract
Objectives:
The present study aims to investigate the therapeutic potential of Cynodon dactylon in alleviating memory deficits associated with Alzheimer’s disease (AD). Specifically, we seek to explore its antioxidant properties and evaluate its potential as an acetylcholinesterase (AChE) inhibitor, with the ultimate goal of identifying C. dactylon bioactives as lead molecules for the management of AD.
Materials and Methods:
We performed an in silico analysis incorporating in silico studies, namely network pharmacology, docking and ADMET profile, to discover the potential effect of C. dactylon L. bioactives against AD targets.
Results:
The present study identified eight bioactive compounds with favourable drug-likeness scores, predicted to target 122 genes involved in crucial pathways. These key targets were involved in 7 pathways with targets such as AChE, butyrylcholinesterase, adenosine receptors A2A, monoamine oxidase (MAO) A, MAOB regulating protein binding, protein dimerisation and serine hydrolase activity. Notably, molecular docking simulations revealed quercetin, kaempferol and luteolin; active ingredients of C. dactylon and exhibited significant binding affinity with AChE.
Conclusion:
These computational insights provide a foundation for further investigation and highlight C. dactylon bioactives as potential candidates for modulating memory deficit in AD, offering new prospects for therapeutic interventions.
Keywords
Cynodon dactylon
In silico analysis
Kaempferol
Luteolin
Quercetin
INTRODUCTION
Memory storage, facilitated by complex neural circuits and neurotransmitter systems of the brain, is crucial to learning and fundamental cognitive processes. Cognitive impairment, defined as a decline in intellectual capacity disrupting daily functioning and relationships, poses significant challenges without extensive impairment of awareness or physical participation.[1] Cognition involves diverse information processing, including memory, perception, language, creative thinking, motivation and skilled movements. The hippocampus, crucial for learning and memory, is a key brain region.[2] Dementia encompasses mental dysfunction, including memory loss, disrupting daily tasks, and impacting the execution of functions.[3] Alzheimer’s disease (AD), the primary cause, manifests with neurofibrillary tangles and insoluble amyloid-beta (Aβ) plaques. These contribute to synaptic loss, neuronal death and cognitive decline.[4] The genetic alteration of amyloid precursor protein is influenced by apolipoprotein E, along with the involvement of beta-site amyloid precursor protein cleaving enzyme 1 (BACE1), presenilin 1, and presenilin 2, plays a significant role in the development of Alzheimer’s disease (AD).[5] The increasing worldwide incidence of dementia is concerning, with projections indicating a significant rise in affected individuals.[6] The current pharmacological treatments for AD offer minimal symptomatic relief and do not address underlying causes. Despite ongoing research, diagnostic capabilities and effective pharmacological interventions are limited. Thus, there is a critical need to explore alternative treatments capable of targeting the multifaceted brain alterations associated with AD, potentially slowing or reducing disease progression.[7,8]
AD’s complexity stems from amyloid plaque deposition, neurofibrillary tangle formation, mitochondrial damage, oxidative stress, neuroinflammation and glutamate excitotoxicity. Hyperphosphorylation of a protein name tau result in synapse loss as well as neuronal dysregulation. The current AD drugs, such as cholinergic activators and glutamate antagonists, offer partial cognitive improvement but entail variable side effects. Acetylcholinesterase (AChE) inhibitors, like donepezil, provide an alternative by enhancing cholinergic transmission and improving cognition. However, their protective effects are limited due to peripheral cholinergic side effects on vital organs, possibly requiring long-term therapy for sustained benefits.[9,10]
Despite scientific advances in receptor expression and nanotechnology, many cognitive disorders lack effective treatments. Network pharmacology, integrating molecular docking and ADMET profiling, addresses complex diseases by targeting underlying pathogenesis through specific drug action mechanisms. This approach provides valuable insights into therapeutic targets and optimises the molecular mechanisms of various drugs, including traditional medicines.
Bioactives of plant origin showcase a broad spectrum of therapeutic effects, capturing the interest of researchers seeking harness in the development of molecules for curing AD. Some of the bioactives such as resveratrol, berberine, aporphine, carnosol, coumarin, flavones, chalcone, neoflavonoid and ginkgolide have been notified as neuroprotective in AD management.[11]
Cynodon dactylon L., commonly known as Doob or Bermuda grass, has a rich history of traditional medicinal use. Recognised as a brain-and-heart tonic in the Unani system,[12] its benefits extend to conditions such as haemorrhages, dysentery, wounds and urinary disorders.[13] Despite this traditional knowledge, scientific exploration of its potential for treating cognitive impairment remains limited. Given the constraints of current AD therapies and C. dactylon’s traditional use as a brain tonic, alternative treatments for memory deficits are needed. C. dactylon displayed a defensive role in aluminium and carbofuran-induced neurotoxicity in rats by lowering lipid peroxidation levels.[14]
This study investigates the molecular mechanisms of C. dactylon L. using a systems biology approach, integrating network pharmacology, molecular docking and ADMET profiling. The goal is to explore its potential therapeutic applications for neuronal dysfunction, particularly for the management of AD.
MATERIALS AND METHODS
The study involved mining phytoconstituents of C. dactylon, assessing their drug-likeness, identifying AD-related targets, conducting gene-set enrichment analysis, preparing ligands and proteins, performing ligand-protein docking and predicting pharmacokinetic and toxicological parameters using various freely accessible databases and computational software tools.
C. dactylon L. phytocompound mining and drug-likeness assessment
The phytoconstituents information of the ‘C. dactylon’ was obtained from earlier scientific reports, Duke’s database and ‘Chemical Entities of Biological Interest’ (ChEBI). For each bioactive, molecular formula, canonical SMILES, molecular weight, hydrogen bond acceptors and donors were collected from the database ‘PubChem’ as shown in [Supplementary Tables 1 and 2]. In addition, the ‘drug-likeness property’ of each bioactive was foreseen using the model Molsoft, with the support of the ‘Lipinski rule of five’.[15]
Target identification
To gather AD targets, the ‘Therapeutic Target Database (TTD)’ was utilised. For each protein and gene ID with ‘Homo sapiens’ as the standard reference, UniProt was used for collecting the functional information of relevant proteins. Phytocompounds that exhibited druggable features were identified based on their canonical SMILES. These SMILES representations were then submitted to the ‘Find my compound Targets’ tool, available on the web server Binding_DB. The tool helped in identifying targets for each phytocompound with a percentile score of ≥70%. After the prediction of targets for the compounds, the identified targets were cross-referenced with the approved therapeutic proteins deposited in the TTD, resulting in the collection of AD-related targets.[16]
Gene-set enrichment and network analysis
The pathogenesis of AD involves changes in protein targets, which can be influenced and modulated by specific phytocompounds. To explore the potential interactions between phytocompounds and protein targets, the ‘STRING’ database was utilised. This allowed for the speculation of interactions between selected phytocompounds and protein targets relevant to AD. Furthermore, the ‘KEGG’ database was employed for collecting information on associated pathways of identified protein targets and phytocompounds. To visually represent and analyse the relationships between phytocompounds, protein targets and enriched pathways, a ‘compound-gene-pathway’ network was built with the help of Cytoscape 3.6.1 software. The grids were analysed using the ‘Network Analyzer’ tool within Cytoscape, treating the connections as ‘direct’ interactions. This analysis likely provided insights into the strength and nature of the relationships between the phytocompounds, protein targets and pathways of AD.
For network visualisation, interactions between gene and phytocompounds were revealed through edge counting, showing the number of connections and potential effects each phytocompound had on its corresponding protein targets. This information would be valuable in understanding the complex relationships between the selected phytocompounds, targets and their related pathways of AD pathogenesis.[17]
Ligand preparation
In the study, the PubChem chemical database was utilised to obtain the 2D and 3D structure of selected ligands. In addition, the canonical SMILES representation of each ligand was also retrieved. To visualise the ligands and examine their 3D structures, the Discovery Studio 2017 (DSV 2019) software was employed. Using DSV 2019, each ligand was visualised, and the resulting structures were saved into the Protein Data Bank (PDB) format.
To optimise and refine the 2D structures of the ligands, the Marvin Sketch software was used. Specifically, the ‘Merck Molecular Force Field 94’ force field was applied to minimise the 2D structures of each ligand. The minimisation process helps in achieving a stable and energetically favourable conformation for further analysis and simulations as shown in Figure 1. Overall, the combination of these tools and techniques enabled the researchers to visualise and prepare the ligands for subsequent analyses and investigations in the study.
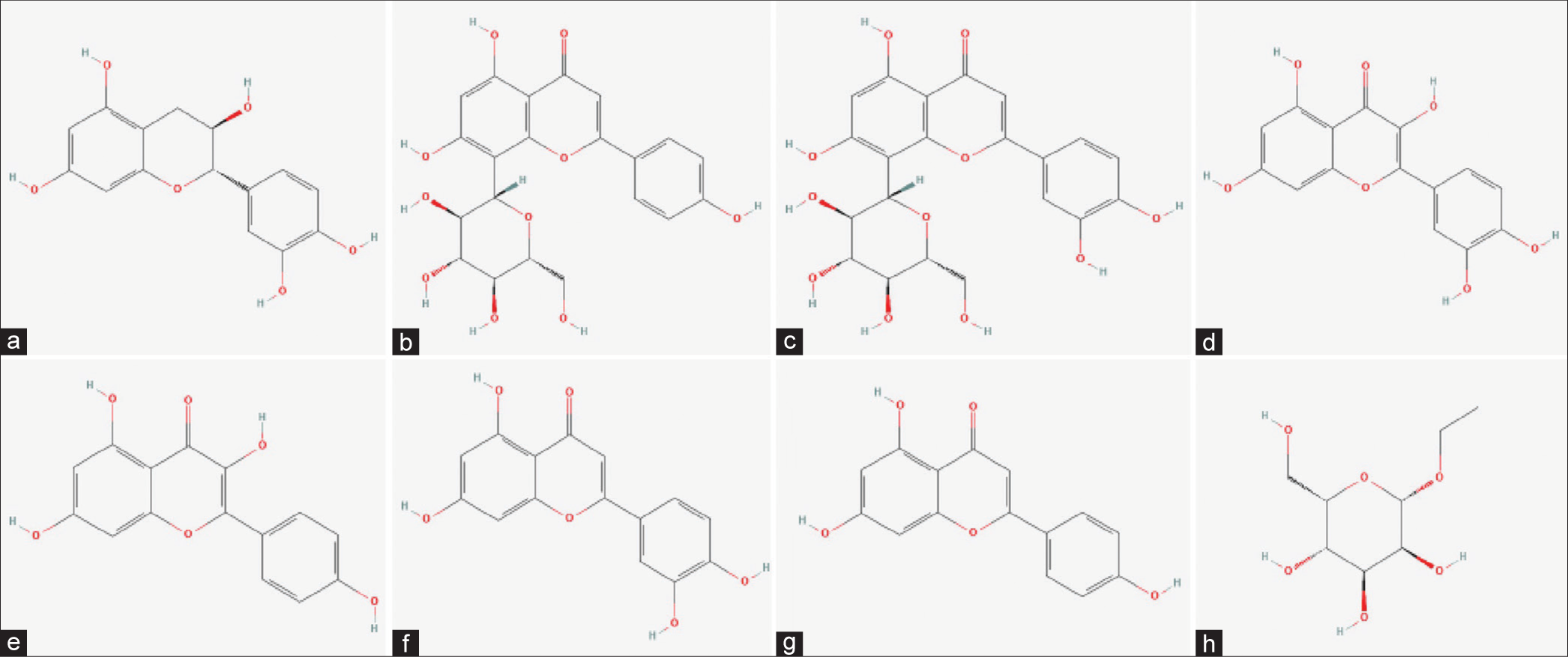
- 2D chemical structure of Cynodon dactylon bioactives (a) catechin, (b) vitexin, (c) orientin, (d) quercetin, (e) kaempferol, (f) luteolin, (g) apigenin, and (h) ethyl beta-D-glucopyranoside.
Protein preparation
The PDB database had been chosen for retrieving the 3D-crystallographic structure of ‘Acetylcholinesterase’ (PDB ID: 4PQE PDB ID: 4EY7). Water molecules and heteroatom were removed using DSV 2017. To assess the quality of protein pockets; onilne servers viz, Procheck, CastP, and Errat were utilized. A protein pocket was selected to dock against the ligand molecule. To comprehend ϕ and ψ scatter of amino acid residues of protein, the Ramachandran plot was opted as shown in Figure 2. In the study, the RCSB-PDB database was selected to retrieve the 3D crystallography of the AChE, identified by its PDB ID: 4PQE. and PDB ID: 4EY7. and 4EY7. To prepare the protein structure for further analysis, water molecules and heteroatoms were detached from the structure by Discovery Studio 2017 (DSV 2017) software. The protein pocket, where the ligand molecule would potentially bind, was analysed using several tools. Procheck, CastP and Errat, which are online servers, were used to assess the quality and integrity of the protein structure. These tools evaluate various aspects of the protein, such as geometry, packing and overall structural quality. Once the protein pocket was identified and validated, it was chosen as the binding site to dock the ligand molecule. Ligand docking is a computational technique that predicts how the ligand interacts with the protein and its potential binding modes.
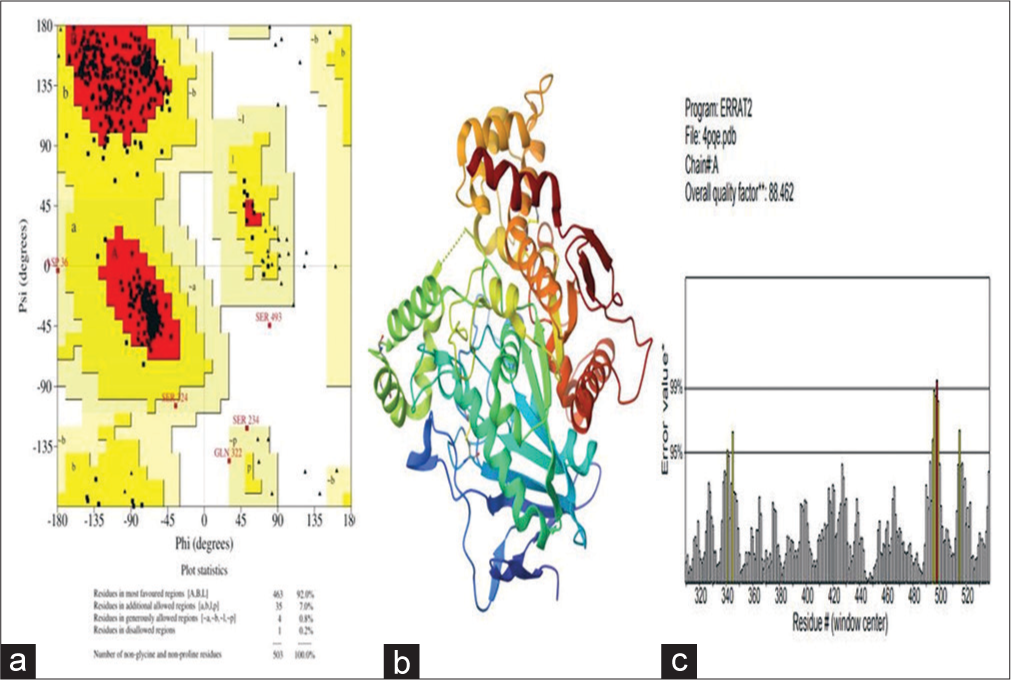
- (a) Acetylcholinesterase (AChE) Ramachandran plot, (b) 3D structure of AChE with its pocket, and (c) Quality of AChE protein.
To understand the conformational tendencies of the amino-acid residues in the protein, the Ramachandran plot is a specific tool which was applied. In this plot, phi (ϕ) and psi (ψ) angles represent rotations around specific bonds in the peptide backbone. The ϕ angle reflects spin between the nitrogen atom of the peptide bond and the α- carbon atom in the amino-acid residue, while ϕ angle reflects the rotation between the alpha carbon atom and the carbonyl carbon atom of the peptide bond. Together, these angles indicate the conformational state of the peptide backbone at each specific residue, which provides insights into their allowed and forbidden regions of conformational space and structural flexibility. Figure 2 likely presents a Ramachandran plot, showing the distribution of ϕ and ψ angles for amino acid residues in the AChE structure.
Overall, these analyses and techniques contribute to an improved understanding of protein-ligand interaction and the structural characteristics of the protein, allowing researchers to gain insights into its functional properties and potential applications in drug discovery or other relevant fields.
Ligand–protein docking
In the study, ‘Autodock Vina’ was utilised for ligand-protein docking analysis to study interactions between phytocompounds present in C. dactylon and protein molecules. Here is an overview of the steps involved: Retrieval of Ligand Structures: The 3-D representation of phytocompounds was transformed in .sdf format, which was taken from the PubChem database. Conversion of Ligand Structures: The obtained.sdf files of phytocompounds were changed to.pdb format with the help of Discovery Studio 2019 software. This conversion step is necessary to prepare the ligands for docking. Retrieval of Protein Structures: X-ray crystallographic structures of protein molecules were acquired from the RCSB in.pdb format. Preparation of Protein Structures: The protein structures were prepared by importing them into the PyRx 0.8v software, where they were converted to the.pdbqt format. This format is required by AutoDock Vina for docking calculations. Docking Parameters: The docking was performed using AutoDock Vina with default grid box settings. Exhaustiveness, which determines the thoroughness of the search, was set to 8 for more accurate results.
Docking calculation
AutoDock Vina calculated using the binding energy of the ligand-protein complexes, indicating the strength of the relations between ligands and target proteins.
Visualisation of results
DSV_2019 was used to confirm the visualisation of the lowest BE of ligand-protein complexes. This allowed for the analysis and interpretation of the docking results, identifying potential binding modes and interactions between the phytocompounds and the target proteins. By performing ligand-protein docking with AutoDock Vina and visualising the results, the study aimed to gain insights into the potential binding interactions and affinities of the phytocompounds from C. dactylon with the target proteins, which could be relevant for understanding their therapeutic potential.[18] Donepezil was considered as a standard molecule to validate the results.
Prediction of pharmacokinetic (ADME) and toxicological parameters
‘PreADMET’ was selected to envisage ADME as well as toxicology-related markers, namely Blood Brain Barrier (BBB) (CBrain/CBlood), intestinal absorption, plasma protein binding, and mutagenic and carcinogenic effects based on the structure of compounds.
Statistical analysis
In the in silico pharmacology, Gene count and false discovery rate interpret protein-protein interactions regulated by the KEGG pathway. Based on the edge count phytocompound, protein-pathway interactions were evaluated. For the molecular docking study, binding energy (kcal/mol) and H-bond interaction/s interpret lead hits specific activity.[19]
RESULTS
Targets prediction and drug-likeness score of C. dactylon bioactives
With the support of phytochemical interaction databases, ChEBI and Dr Duke’s datasets, 75 phytochemicals were identified from C. dactylon; only 8 out of them showed optimistic drug-likeness score (Catechin scored uppermost, i.e., 0.64) as shown in Table 1. These active compounds targeted 122 genes predicted from binding database [Supplementary Table 3]. Peer interpretation recognised 3 compounds to target 5 proteins linked with AD [Table 2].
| Compounds | Pubchem ID | Molecular Weight (g/mol) | HBD | HBA | Log P | Drug-likeness score |
|---|---|---|---|---|---|---|
| AcceptableValue | - | < 500 | <5 | < 10 | <5 | <1 |
| Catechin | 9064 | 290.27 | 5 | 6 | 0.53 | 0.64 |
| Vitexin | 5280441 | 432.11 | 7 | 10 | 0.77 | 0.6 |
| Orientin | 5281675 | 448.1 | 8 | 11 | 0.33 | 0.59 |
| Quercetin | 5280343 | 302.23 | 5 | 7 | 1.19 | 0.52 |
| Kaempferol | 5280863 | 286.28 | 4 | 6 | 1.61 | 0.5 |
| Luteolin | 5280445 | 286.05 | 4 | 6 | 2.78 | 0.38 |
| Apigenin | 5280443 | 257.11 | 1 | 3 | 2.68 | 0.29 |
| Ethyl-α-D glucopyranoside | 11127487 | 208.09 | 4 | 6 | -1.79 | 0.01 |
Log P: Octanol/Water partition coefficient; HBD: Hydrogen Bond Donor; HBA: Hydrogen Bond Acceptor.
| Compound | Compound type | Gene ID (compound – target probability score) |
|---|---|---|
| Apigenin | Flavonoid | ACHE (0.93), BCHE (0.86), ADORA2A (0.86), MAOA (0.97), MAOB (0.97), |
| Kaempferol | Flavonoid Amine oxidase (flavin-containing) A |
ACHE (0.97), BCHE (0.97), ADORA2A (0.97), MAOA (0.97), MAOB (0.86), BACE1 (0.7) MAOA (0.97) |
| Luteolin | Flavonoid | MAOA (0.9), MAOB (0.9). |
| Quercetin | Flavonoid | MAOA (1), MAOA Amine oxidase (flavin-containing) A (0.92) |
ACHE: Acetylcholinesterase; BCHE: Butylcholinesterase; ADORA2A: Adenosine A2A receptor; MAOA: Mono amine oxidase A; MAOB: Mono amine oxidase B; BACE1: Beta-site APP Cleaving Enzyme 1.
| KEGG ID/Gene Ontology ID | Pathway description | Gene count | False Discovery Rate | Protein targets within the network |
|---|---|---|---|---|
| hsa01100 | Metabolic pathways | 30 | 1.16E-08 | HSD17B2, ALOX12, NT5E, ARG2, TYR, MECR, SI, AKR1B1, CBR1, NOS3, FASN, GAA, ALOX12B, COX5A, GANAB, MAOA, AKR1B10, PTGS1, PNLIP, ALOX5, ALPL, MAOB, XDH, CYP1A1, ALOX15B, CYP19A1, TST, MGAM, ALOX15, HSD17B1 |
| hsa04726 | Serotonergic synapse | 8 | 1.04E-05 | ALOX12, ALOX12B, MAOA, PTGS1, ALOX5, MAOB, ALOX15B, ALOX15 |
| hsa00330 | Arginine and proline metabolism | 4 | 0.002 | ARG2, NOS3, MAOA, MAOB |
| hsa00350 | Tyrosine metabolism | 3 | 0.0083 | TYR, MAOA, MAOB |
| hsa00380 | Tryptophan metabolism | 3 | 0.0108 | MAOA, MAOB, CYP1A1 |
| hsa00360 | Phenylalanine metabolism | 2 | 0.0234 | MAOA, MAOB |
| hsa00340 | Histidine metabolism | 2 | 0.0317 | MAOA, MAOB |
Gene set-enrichment pathway
The serotonergic synapse, metabolic, arginine, proline, tyrosine, tryptophan, phenylalanine and histidine metabolisms were among the pathways that may have contributed to the pathophysiology of AD [Table 3]. The maximum edge count in the network analysis was scored by apigenin, luteolin, quercetin and kaempferol; these substances were recognised as flavonoids to target monoamine oxidase (MAO) A and MAOB [Figure 3].
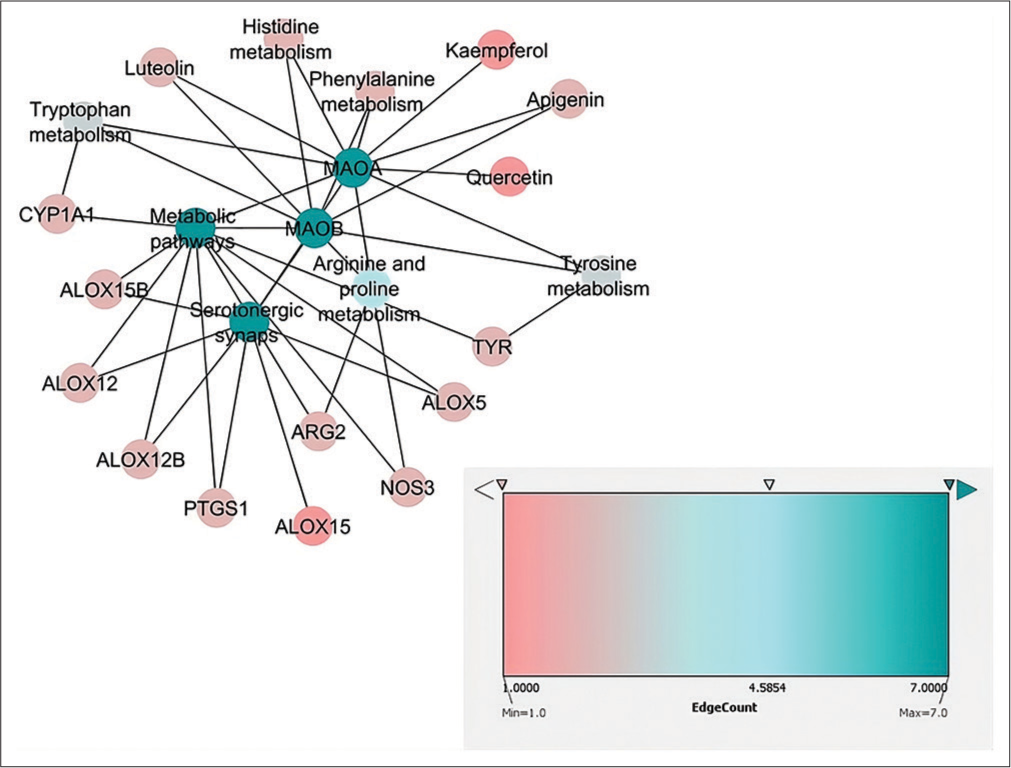
- Network representation of Cynodon dactylon phytocompounds-target proteins pathways.
Gene ontology analysis
The 4 functional pathways, i.e., identical protein binding, AChE activity, protein dimerisation activity and serine hydrolase activity, were identified to target the proteins of interest present in AD pathogenesis. The apigenin, luteolin, kaempferol and quercetin showed the highest edge count score for AChE, butyrylcholinesterase and adenosine receptors A2A (ADORA2A) targets, as shown in Figure 4.
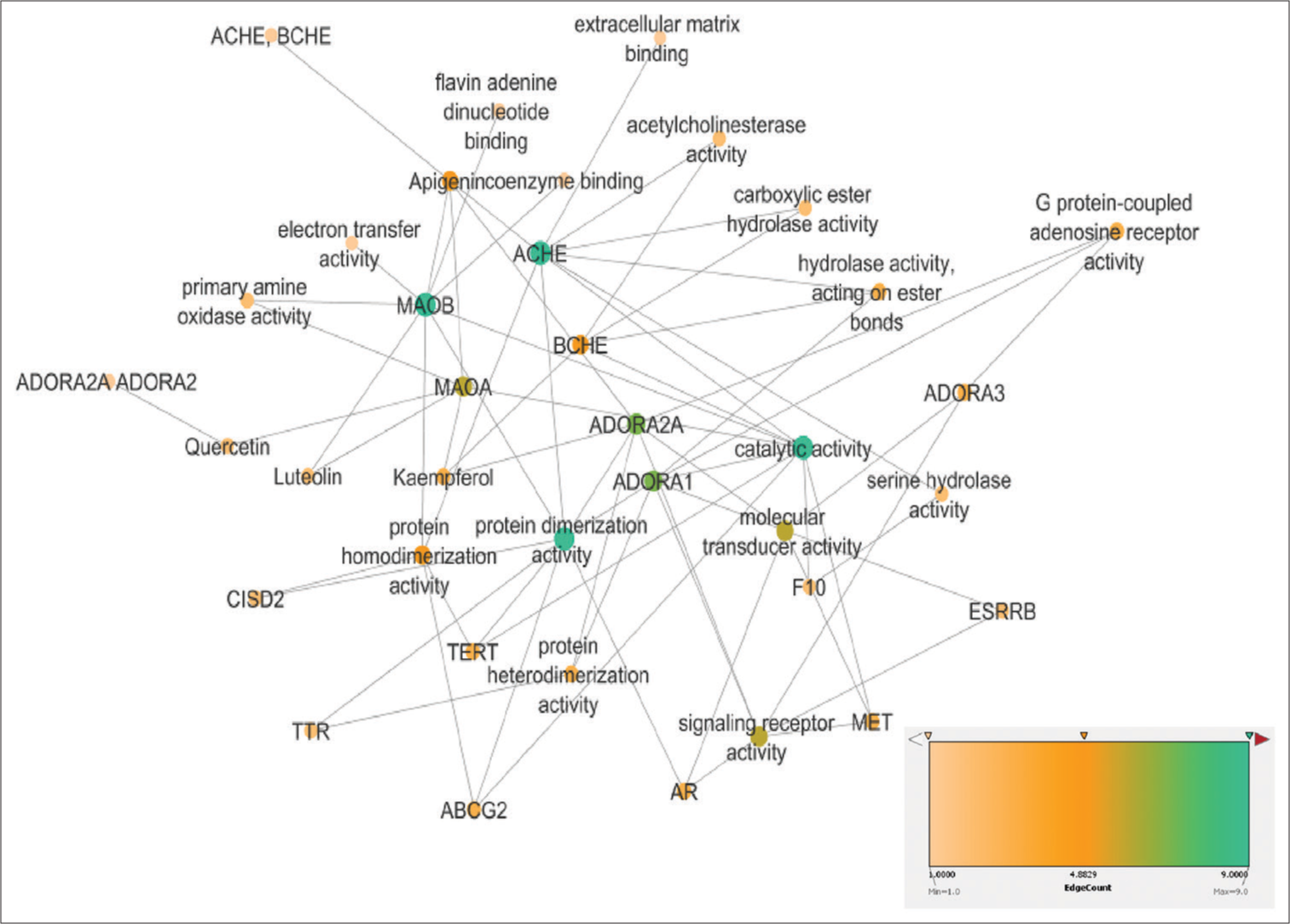
- Gene ontology presentation of Cynodon dactylon phytocompounds-target proteins pathways.
Docking score
Molecular interaction of C. dactylon bioactives with AChE (PDB: 4PQE)
Amongst the three compounds, quercetin scored the highest binding energy (−4.04 kcal/mol), whereas luteolin scored the lowest binding energy (−3.65 kcal/mol). The binding affinity, inhibitory constant and hydrogen bond interaction are represented in Table 4. Luteolin scored the highest hydrogen bond interaction through 3 interactions by amino acid residues, namely ASP36, THR94 and ASP92. However, quercetin and apigenin expressed the lowest H-bond interaction with protein through two H-bond interactions, namely arginase (ARG) 47, ASP 92 and ASP 92, VAL 90, respectively. The interaction of each phytoconstituent with AChE is shown in Figure 5. The donepezil was considered a clinically accepted standard drug, and it was docked with AChE (PDB: 4PQE), scored the highest binding energy (−8.3 kcal/mol), and had 1 hydrogen bond interaction with PHE 297 [Supplementary Figure 1].
| Compounds | Binding energy (Kcal/Mol) | Inhibitory constant (mM) | H bond interaction | No. of hydrogen bond interaction |
|---|---|---|---|---|
| Quercetin | −4.04 | 1.08 | ARG47, ASP92 | 2 |
| Apigenin | −3.82 | 1.52 | ASP92, VAL90 | 2 |
| Luteolin | −3.65 | 2.11 | ASP36, THR94, ASP92 | 3 |
| Donepezil | −8.3 | 1.38 | PHE295 | 1 |
AChE: Acetylcholinesterase
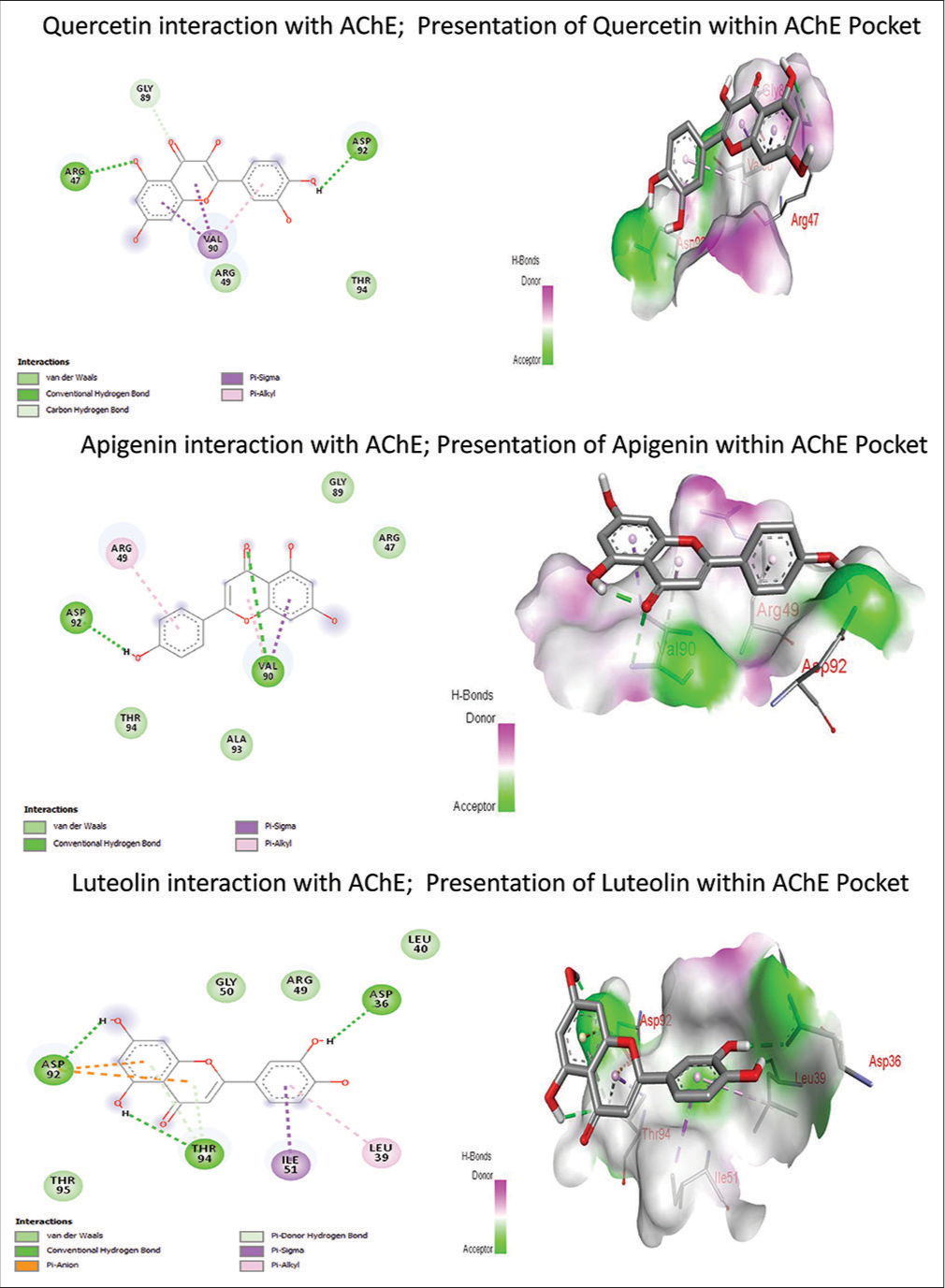
- Molecular docking study of Cynodon dactylon L. phytoconstituents with acetylcholinesterase. AChE: Acetylcholinesterase (PDB ID: 4PQE)
Molecular interaction of C. dactylon bioactives with AChE (PDB: 4EY7)
Another protein of interest, AchE, with PDB ID: 4EY7, was selected to know the molecular interaction confirmations of selected bioactives from C. dactylon. Quercetin showed the highest binding affinity (−9.0 kcal/mol) as compared to donepezil, luteolin, kaempferol, and apigenin (-8.9, −8.7, −8.6, −8.5 kcal/mol, respectively), via forming two hydrogen bonds with HIS381 and ARG525 [Table 5]. The bioactive’s interaction with the 4EY7 pocket is represented in Figures 6a and b.
| Compounds | Binding energy (Kcal/Mol) | Inhibitory Constant (mM) | H Bond interaction | No. of Hydrogen bond interaction |
|---|---|---|---|---|
| Quercetin | -9.0 | 1.53 | HIS381, ARG525 | 2 |
| Luteolin | -8.7 | 2.11 | HIS381, ARG525 | 2 |
| Kaempferol | -8.6 | 1.89 | ARG525, HIS381 | 2 |
| Apigenin | -8.5 | 1.08 | HIS381, ARG525 | 2 |
| Donepezil | -8.9 | 1.41 | HIS381 | 1 |
AChE: Acetylcholinesterase, PDB: Protein Data Bank
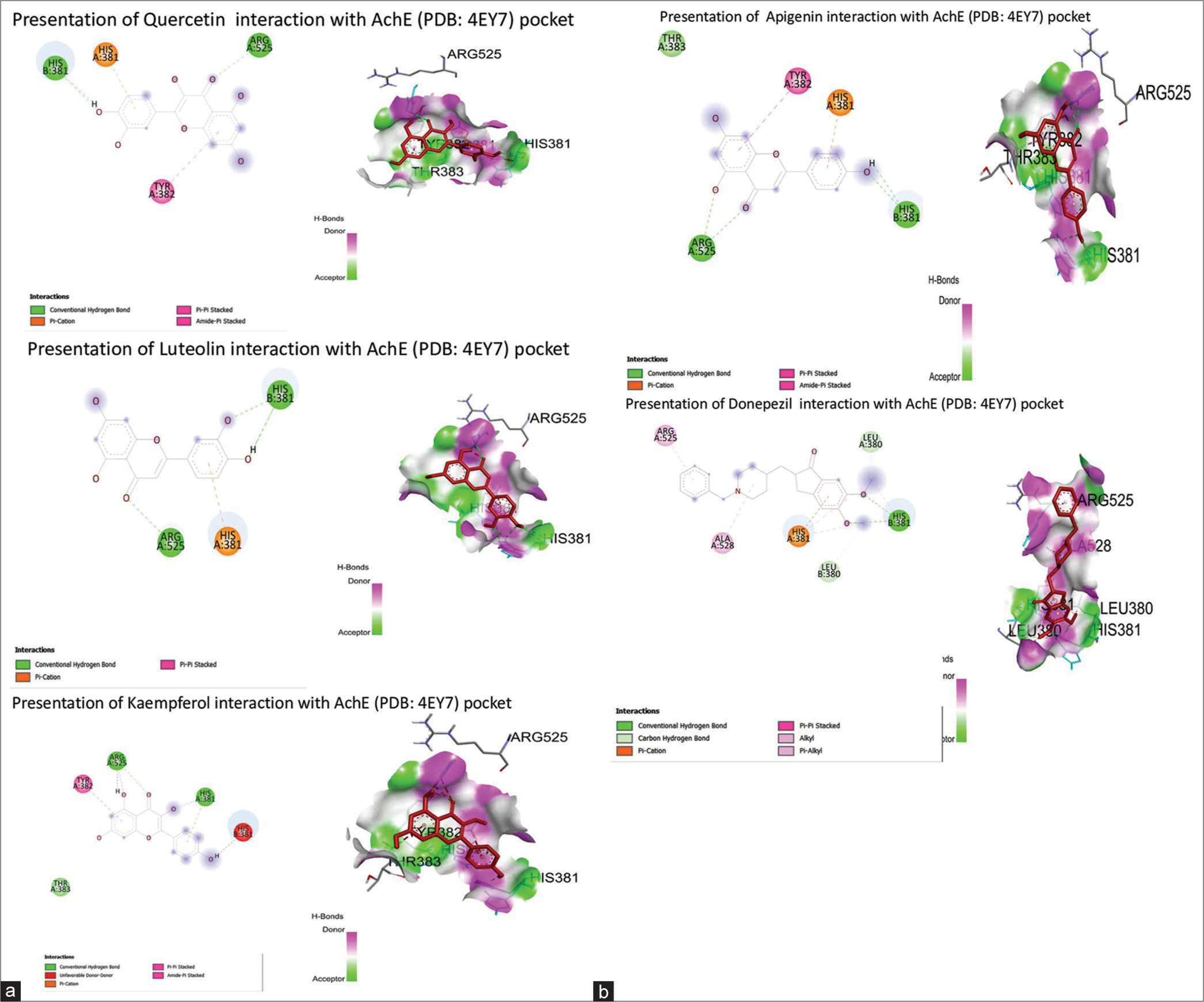
- (a and b) Presentation of Cynodon dactylon bioactives interaction with acetylcholinesterase ( Protein Data Bank ID: 4EY7) pocket. AChE: Acetylcholinesterase.
Molecular interaction of C. dactylon bioactives with MAO (PDB: 2Z5X)
Out of four phytoactives, kaempferol showed the highest binding energy (−8.7 kcal/mol) with one hydrogen bond attachment at ASP 132. Apigenin and quercetin showed the lowest binding energies through single hydrogen bonds, i.e., HLS187 and PRO113, respectively. However, luteolin expressed binding affinity (−6.9 kcal/mol) with 2Z5X through two hydrogen bonds (GLU 477, LYS 440). These were compared with donepezil, which showed a docking score (−7.9 kcal. mol), as shown in Table 6. The bioactive’s interaction with the 2Z5X pocket is represented in Figures 7a and b.
| Compounds | Binding energy (Kcal/Mol) | H Bond interaction | No. of Hydrogen bond interaction |
|---|---|---|---|
| Kaempferol | -8.7 | ASP132 | 1 |
| Apigenin | -7.5 | HIS187 | 1 |
| Quercetin | -7.1 | PRO113 | 1 |
| Luteolin | -6.9 | GLU277, LYS440 | 2 |
| Donepezil | -7.9 | ARG76 | 1 |
PDB: Protein Data Bank, MAO: Monoamine oxidase
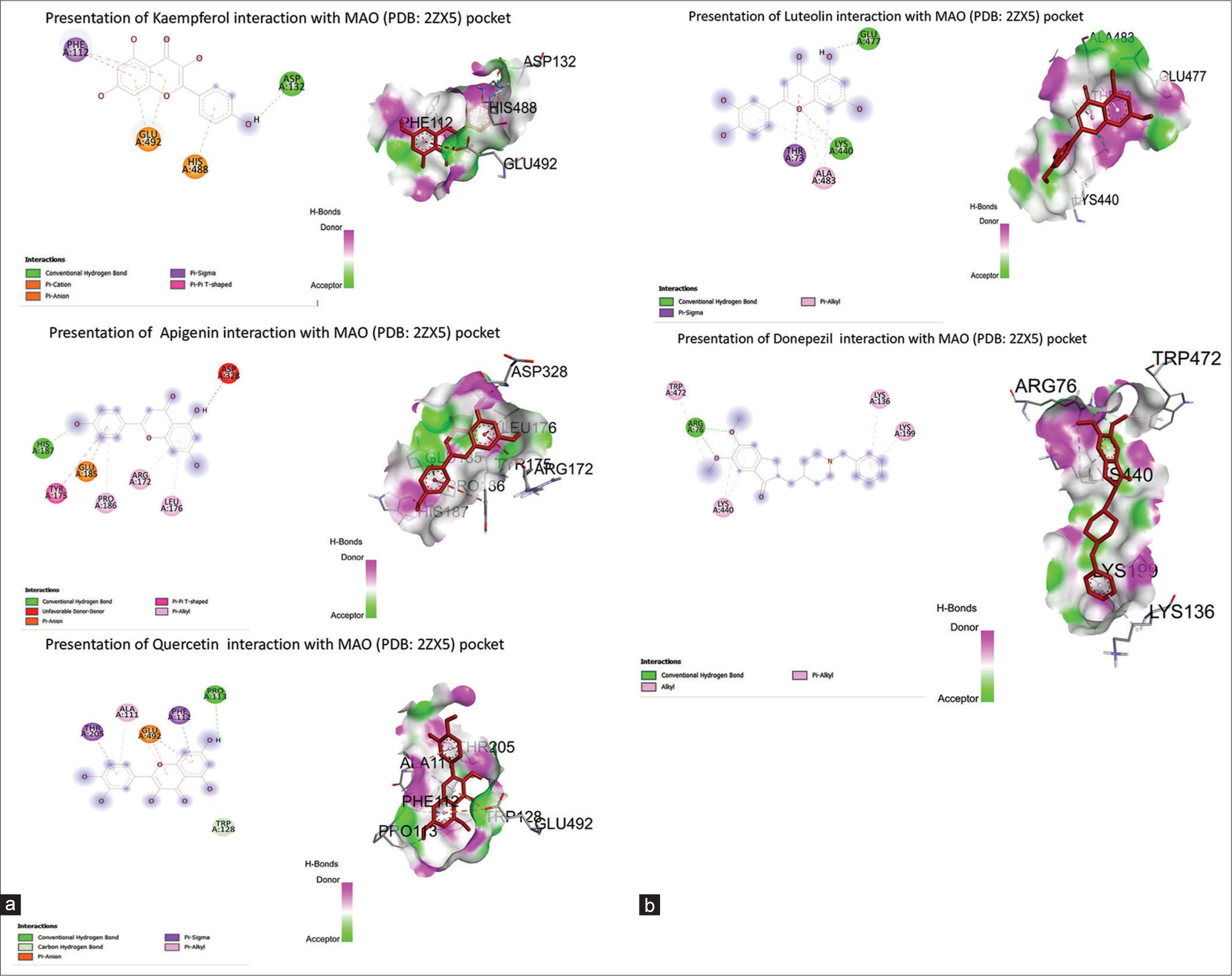
- (a and b) Presentation of Cynodon dactylon bioactives interaction with monoamine oxidase (Protein Data Bank ID: 2Z5X) pocket. AChE: Acetylcholinesterase
ADMET analysis
The selected eight compounds showed 14–88% of human intestinal absorption, 60–100% plasma protein binding except ethyl D-glucopyranoside (10%) and CYP2C19, CYP2C9, CYP3A4 inhibitors except ethyl D-glucopyranoside. All the compounds were non-inhibitors of CYP2D6. Apart from orientin, all the other bioactives were considered mutagens and had a low to high risk of human Ether-a-go-go-Related Gene (hERG) inhibition. All the compounds did not show carcinogenicity in mice; however, apigenin, vitexin, luteolin, quercetin and kaempferol showed carcinogenicity in rodents. Other ADME and toxicity profiles of eight bioactives are described in Table 7.
| Compounds | ||||||||
| ADME | Apigenin | Vitexin | Orientin | Luteolin | Ethyl D-glucopyranoside | Quercetin | Kaempferol | Catechin |
| BBB (logBB) | 0.565113 | 0.038527 | 0.0340797 | 0.367582 | 0.108799 | 0.172765 | 0.286076 | 0.394913 |
| Buffer solubility (mg/L) | 75.3288 | 196.168 | 562.676 | 220.694 | 60375.8 | 64.4795 | 22.0776 | 2253.95 |
| Caco2 (nm/sec) | 10.5468 | 5.48785 | 2.99553 | 4.53973 | 2.2918 | 3.4129 | 9.57744 | 0.656962 |
| CYP2C19 | I | I | I | I | I | I | I | I |
| CYP2C9 | I | I | I | I | I | I | I | I |
| CYP2D6 | NI | NI | NI | NI | NI | NI | NI | NI |
| CYP3A4 | I | I | I | I | NI | I | I | I |
| HIA (%) | 88.1228 | 31.3741 | 14.9885 | 79.4272 | 38.1563 | 63.4852 | 79.4392 | 66.7079 |
| P-gp | NI | NI | NI | NI | NI | NI | NI | NI |
| PPB(%) | 97.25 | 61.32 | 63.12 | 99.71 | 10.83 | 93.23 | 89.60 | 100 |
| Water solubility (mg/L) | 159.88 | 344.56 | 256.35 | 121.50 | 5489 | 96.43 | 127.30 | 1240.55 |
| Toxicological Parameters | ||||||||
| Ames test | M | NM | NM | M | M | M | M | M |
| Carcinogenecity (Mouse) | -ve | -ve | -ve | -ve | -ve | -ve | -ve | -ve |
| Carcinogenecity (Rat) | +ve | -ve | -ve | +ve | -ve | +ve | +ve | -ve |
| hERG Inhibition | MR | HR | HR | MR | LR | MR | MR | MR |
| FAT (medaka) | 0.0280 | 1.0581 | 1.3892 | 0.0329 | 242.117 | 0.0778 | 0.0642 | 0.0608 |
| FAT (minnow) | 0.0152 | 0.7631 | 0.8645 | 0.0169 | 114.34 | 0.0335 | 0.0294 | 0.03614 |
BBB: Blood-Brain Barrier, CaCO2 p: Predicted value of intestinal absorption through CaCO2, BS: Buffer Solubility, hERG Inhibition: Predicted result of hERG inhibition by compounds. hERG inhibition leading to QT prolongation and cardiac risk., P-gp: P-glycoprotein, PPB: Plasma Protein Binding, HIA: Human Intestinal Permeability, FAT: Fish Aqueous Toxicity, I: Inhibitor, NI: Non-Inhibitor, M: Mutagen, MR: Medium Risk, HR: High Risk, LR: Low Risk.
DISCUSSION
Aspects in the contemporary landscape of drug discovery for novel compounds, traditional herbs are being investigated through sophisticated pharmacological approaches, integrating advanced techniques such as bioinformatics and polypharmacology. These methodologies provide a strategic framework for research validations, as in silico studies have the capability to identify multiple disease pathways influenced by numerous targets, assessing both the therapeutic and adverse effects of drugs through the utilisation of specialised tools.
Utilising drug-likeness properties, we unveiled diverse associations between proteins and compounds by subjecting the phytoconstituents of C. dactylon to interactions with various proteins. Subsequently, these interactions were subjected to further analysis, enabling the prediction of potential targets for the phytoconstituents through the application of the TTD.
In this investigation, five compounds – namely, apigenin, luteolin, kaempferol, quercetin and tyrosine – were identified as modulators targeting ten therapeutic protein targets associated with AD. These protein targets include monoamine oxidase (MAO-A, MAO-B), cytochrome P450 1A1 (CYP1A1), arachidonate lipoxygenases (ALOX5, ALOX12, ALOX12B, ALOX15, ALOX15B), arginase (ARG2) and nitric oxide synthase (NOS3). In addition, the study revealed that metabolic pathways such as tryptophan, histidine, phenylalanine, tyrosine, arginine and proline metabolisms and serotonergic pathways play significant roles in AD.
Indeed, according to existing literature, MAO is an enzyme accountable for catalysing the oxidative damage to various catecholamines. This enzyme has two isomers, namely MAO-A primarily acts on substrates such as serotonin, dopamine, noradrenaline and adrenaline, and MAO-B targets substrates such as tyramine and phenylalanine.
The significance of MAO in neurobiology is noteworthy. An increase in the activity can lead to elevated oxidation of neurotransmitters. This heightened oxidation, in turn, reports downregulating quantities of neurotransmitters. Such neurochemical changes have been connected with the advancement of neurodegenerative disorders. The intricate relationship between MAO activity, neurotransmitter levels and the progression of these disorders underscores the importance of understanding the role of MAO in neurodegenerative processes.[20]
Certainly, the flavone apigenin, abundantly found in various herbs, is recognised as an aglycone of several naturally occurring glycosides. In a computer-based study, a potential molecular interaction was identified between a specific isoform of apigenin, namely 6-prenyl apigenin and MAO-A active sites. Moreover, the complex affinity of 6-prenyl apigenin to MAO-A active site was observed to be notably high, suggesting its capability to inhibit the enzyme.
These findings suggest that 6-prenyl apigenin holds promise as a prime molecule for the development of new inhibitors. The identified molecular interaction and high binding affinity underscore its potential therapeutic significance in the synthesis of novel compounds targeting MAO-A, which could have implications in the development of treatments for conditions associated with its dysregulation, including neurodegenerative disorders.
Apigenin’s cognitive-enhancing effects are likely mediated through its multifaceted actions on the brain-derived neurotrophic factor (BDNF)/tropomyosin-related kinase B pathway, amyloidogenesis and apoptosis signalling pathways. By promoting neurotrophic support, reducing Aβ production and preventing neuronal apoptosis, apigenin shows promise as a natural compound for potential therapeutic interventions in conditions linked to cognitive decline.[21]
Treatment with synthetic apigenin (100 and 200 mg/kg/day) was revealed to enhance cognitive function, as proven by behavioural tasks (MWM, T maze and NORT). Adding to this separate study reported the docking ability of apigenin with AChE, a key enzyme involved in acetylcholine (ACh) breakdown.
Apigenin (25 mg/kg) was given to healthy 7-week-old mice for 10 days, and this increased their recall of information, which was tested using a Morris water maze and increased neurogenesis by promoting neuronal differentiation in the hippocampus.[22]
Researchers claimed anti-inflammatory effects of apigenin by the mechanism of down-regulating cytokines as well as nitric oxide (NO) release and reduction of apoptosis mediated by caspase 3/7 in induced pluripotent stem cell-derived neurons in AD patients.[23]
An additional investigation revealed that lipopolysaccharide-stimulated microglial cells were mediated by two essential tryptophan metabolic enzymes: Indoleamine 2, 3-dioxygenase and 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase. This stopped the production of inflammatory metabolites in a vicious loop and stopped neurodegeneration. The potential mechanism of apigenin’s anti-inflammatory action involves impeding pro-inflammatory mediators through the deactivation of the nuclear factor kappa B, Jun N-terminal kinase mitogen-activated protein kinase and extracellular signal-regulated kinase pathways.[24] The present study does co-relate with the enrichment analysis of apigenin targeting tryptophan metabolism, as mentioned in Table 3.
MAO enzymes play a pivotal role in serotonin degradation and targeting the tryptophan metabolism pathway presents a potential strategy to inhibit MAOenzyme actions, leading to increased serotonin synthesis for neuroprotection. Flavonoids have the potential to prevent neurodegenerative diseases such as AD because of their ability to pass the blood-brain barrier. AD, characterised by elevated ‘Amyloid-β’ proteins along with oxidative stress, quercetin’s antioxidant capabilities become crucial. By inhibiting the generation of reactive oxygen species (ROS) and preventing fibril/senile plaque formation of amyloid proteins, quercetin demonstrates neuroprotective actions, mitigating cell damage and inflammatory processes. Extensively studied by in vivo and in vitro paradigms, quercetin emerges as a promising target for inhibiting the MAO-A enzyme, which is involved in the pathogenesis of dementia.[25]
Network image expresses a direct connection between kaempferol and MAO-A. In vitro results have revealed that kaempferol acts as an MAO inhibitor where MAO-A inhibition was greater than MAO-B inhibition. Therefore, kaempferol can be targeted to inhibit the effects of MAO-A in the pathogenesis of AD.
Luteolin, a flavonoid compound directly linked to MAO enzymes, exhibits inhibitory actions on MAO-A; in animal models, luteolin has demonstrated its protective effects against AD by crossing BBB and reducing A-β levels. This reduction is attributed to its inhibition of β and γ-secretase, key enzymes in the generation of amyloidogenic A-β. In addition, luteolin mitigates the phosphorylation of protein tau, the development of neurofibrillary tangles, and the deposition of A-β, providing comprehensive neuroprotection.[26]
Furthermore, luteolin’s administration in animal models has been shown to enhance brain insulin sensitivity and decrease neuroinflammation, contributing towards protection against AD.[27] Luteolin’s treatment reversed the scopolamine induced suppression of neuroblast differentiation and cell proliferation in the dentate gyrus. Mechanistically, amelioration of amnesia by luteolin may be linked to increased BDNF, ACh and modulation of lipid peroxidase (LPO).[20]
Considering these findings, it is noteworthy that tyrosine, a precursor for catecholamines (dopamine, adrenaline/epinephrine and noradrenaline/norepinephrine), could be a possible target for MAO inhibition. The reduction of catecholamine levels in AD, credited for the loss of noradrenergic neurons and metabolism by MAO-A and MAO-B enzymes, suggests a promising avenue for intervention through tyrosine metabolism.[28]
In a docking study, it was found that apigenin has anti-AChE properties and was found to inhibit the activity of the AChE enzyme. Therefore, apigenin can be a promising compound to target and inhibit the AChE enzyme, thereby increasing the levels of ACh in AD pathogenesis.
In the field of molecular docking and computational drug design, the selection of target proteins and bioactive compounds is critical for identifying potential therapeutic interactions. In this context, two proteins of interest are AChE with PDBID: 4PQE and PDB ID: 4EY7. The interaction of these proteins with selected bioactive compounds from C. dactylon, namely quercetin, apigenin, kaempferol and Luteolin, has been studied to evaluate their binding affinities and potential as drug candidates.
The human AChE structure with PDB ID: 44EY7, when docked with the bioactive compounds’ quercetin, apigenin and luteolin, exhibited the lowest binding energy scores amongst the evaluated proteins. This implies that these compounds form highly stable complexes with the 4EY7 structure. The low binding energy is indicative of significant interactions, such as hydrogen bonding, lipophilic interactions as well as van der Waals forces that contribute to stability and affinity towards complex which was compared with earlier reports.[29]
Conversely, the interaction of the selected bioactives with the human AChE structure with PDB ID: 4PQE showed the highest docking scores. Higher docking scores indicate weaker binding affinities compared to those observed with 4EY7. This suggests that the bioactive compounds may not form as stable complexes with the 4PQE structure, which could be due to differences in active-site conformation or overall protein moiety affecting ligand accommodation.
MAO inhibition plays a critical role in managing neurological disorders. MAO inhibitors can prevent the breakdown of neurotransmitters, thereby enhancing their availability and ameliorating symptoms associated with these conditions.
In our study, we focused on identifying potential MAO inhibitors from natural sources. Specifically, we selected flavonoids from C. dactylon, a plant known for its medicinal properties. The integration of docking techniques with structural data from PDB ID: 2Z5X provides a powerful approach for evaluating the binding affinities of flavonoids with MAO. The insights gained from these studies could inform future drug development efforts aimed at targeting MAO, ultimately contributing to the management of neurodegenerative diseases.
The flavonoids tested included kaempferol, apigenin, quercetin and luteolin. Molecular docking results revealed that kaempferol exhibited the highest binding scores among the tested compounds. This suggests that kaempferol has the strongest potential to interact with the active site of the MAO enzyme, making it a promising candidate for further investigation as an MAO inhibitor.
Kaempferol’s superior binding affinity compared to apigenin, quercetin and luteolin indicates that its structural features may allow for more effective interactions with key residues in the MAO active site. This finding highlights the potential of kaempferol as a lead compound in the progress of newer therapeutic agents aiming at neurological disorders through MAO inhibition.
Adenosine receptors ADORA2A and ADORA2 exhibit high expression levels in the basal ganglia of the brain, with studies including Meng et al. indicating their abundant presence in AD and their significant role in the progression of AD.[30]
Considering this, quercetin and apigenin emerge as potential targets for reducing the expression of ADORA2A and ADORA receptors. These compounds could serve as promising antagonists to modify the action of adenosine receptors, potentially mitigating the adverse effects allied with AD progression. Inhibition of such receptors by quercetin and apigenin opens avenues for therapeutic strategies intended at averting or slowing down the cognitive decline and pathological processes characteristic of AD.
Oxidative stress may be from various pathogical environments, in which ALOX and its subtype enzymes play pivotal role and this was also observed in clinical conditions of Alzhiemer’s disease. This oxidative stress adds to the pathology of AD by promoting lipid peroxidation in the brain. Addressing this, targeting the genetic deletion of ALOX enzymes emerges as a probable strategy to reduce cellular oxidative stress in Alzheimer’s brains. The rationale behind this approach is based on the understanding that inhibiting the ALOX metabolic pathway, which contributes to neurodegeneration, could restore the oxidative balance in AD.
The prior enquiry highlighted the importance of serotonin receptor inhibitor (5-HT6R) in effectively mitigating memory impairment by reducing Aβ levels through inhibiting γ-secretase function and deactivation of astrocytes as well as microglia in mouse models of AD. The assessment involved a Morris water maze, passive avoidance test, western blot analysis, immunohistochemistry and estimation of Aβ1-42, β- and γ-secretase activities.[31] The present results are congruent with enrichment analysis of bioactive from C. dactylon that modulates the serotonin synapse, suggesting a potential correlation.
NO-induced oxidative stress is implicated as a factor in the pathogenesis of AD. NOS facilitates for converting L-arginine to NO. ROS can then interact with NO, forming peroxynitrate, a compound that promotes lipid peroxidation and fastens degenerative processes, which leads to AD. There have been risk conditions applicable to pose late-onset AD which includes polymorphism in NOS3 at Glu298th position. The present study also explores the NOS3 gene involvement in metabolic pathways within the gene enrichment network, which can be correlated with the relevance of reducing the expression of the NOS3 gene emerges as a potential focus for AD.[32]
Furthermore, kaempferol has been demonstrated to significantly elevate antioxidant chemicals such as glutathione and superoxide dismutase while concurrently reducing tumour necrosis factor as well as malondialdehyde. These findings suggest that kaempferol may ameliorate memory impairment in ovariectomised rats induced by streptozotocin, possibly through increasing endogenous glutathione and superoxide dismutase levels presented in the hippocampus, reducing neuroinflammation. A recent report indicates that kaempferol may possess neuroprotective properties against cognitive deficits.[33]
Previous literature suggests that kaempferol and quercetin invoke PI3K/AKT signalling cascade to oppose the Aβ effect alongside reducing hyperphosphorylation of tau at the synapse. The kaempferol inhibits AChE (BE; −10.26 kcal/mol) and BACE1 (IC50; 14.7 µM). The molecular interaction of quercetin with AChE reports BE; −7.9 kcal/mol and inhibits BACE1 5.4 µM, which could enhance neuroplasticity in the AD brain. Another review suggests the role of kaempferol, apigenin, and quercetin have a potential role in MAO inhibition, which can be corroborated with current molecular docking reports[34] as shown in Tables 4 and 5.
The hydroethanolic extract of C. dactylon has been reported in a recent study to ameliorate cognitive impairment in Wistar rats. This improvement is attributed to the restoration of antioxidant levels and the inhibition of the AChE enzyme. The conclusions of this study suggest a potential correlation with in silico studies.[35]
In addition, network pharmacology analyses have identified several phytocompounds, namely apigenin, luteolin, kaempferol, quercetin and tyrosine, which may modulate pathways related to scopolamine-induced AChE activity, oxidative stress, neuroinflammation and monoamine oxidase. This insight into the potential actions of these compounds suggests a multifaceted approach to addressing cognitive impairment involving the regulation of various pathways implicated in neurological function.
The combined evidence from both experimental and computational studies provides a wide-ranging understanding of the potential beneficial effects of C. dactylon along with its associated phytocompounds in mitigating cognitive impairment through antioxidant restoration and AChE inhibition.
CONCLUSION
The anti-amnesic activity of C. dactylon appears to be facilitated through the mechanisms of bioactives such as apigenin, kaempferol, luteolin and quercetin. These compounds exert their effects by targeting AChE, MAOB, ADORA2A and ADORA2 through similar protein binding activities. In addition, their actions involve influencing AChE activity, protein dimerisation activity and serine hydrolase pathways associated with AD.
This investigation not only supports conventional use of C. dactylon as brain energiser according to Unani- system of medicine but also provides a molecular understanding of how specific bioactives within C. dactylon may contribute to its anti-amnesic properties. The identified targets, including AChE, MAOB, ADORA2A and ADORA2, were recognised as crucial roles in the pathogenesis of AD. Looking forward, there is potential for isolating bioactives from C. dactylon for further exploration in wet laboratory experiments. This could involve testing the isolated compounds to validate their modulatory effects on the identified pathways, offering a more in-depth understanding of the therapeutic potential in addressing cognitive impairments associated with AD.
Acknowledgement
I sincerely thank the computational tools (Protein Data Bank, PreADMET, Molsoft, Autodock vina, Discovery studio, Cytoscape 2.6.1) and KLE College of Pharmacy, Hubli for providing for providing necessary support in the preparation of manuscript.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required as there are no patients involved in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Antiamnesic activity of an ayurvedic formulation chyawanprash in mice. Evid Based Complement Alternat Med. 2011;2011:898593.
- [CrossRef] [PubMed] [Google Scholar]
- Cognitive deficits in psychiatric disorders: Current status. Indian J Psychiatry. 2006;48:10-20.
- [CrossRef] [PubMed] [Google Scholar]
- Dementia: What pharmacists need to know. Can Pharm J (Ott). 2017;150:118-29.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanisms of Alzheimer's disease pathogenesis and prevention: The brain, neural pathology, N-methyl-D-aspartate receptors, tau protein and other risk factors. Clin Psychopharmacol Neurosci. 2017;15:1-8.
- [CrossRef] [PubMed] [Google Scholar]
- Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995;375:754-60.
- [CrossRef] [PubMed] [Google Scholar]
- World Alzheimer report: The global economic impact of dementia In: Alzheimer's Disease International. 2010.
- [Google Scholar]
- Morphological criteria for the recognition of Alzheimer's disease and the distribution pattern of cortical changes related to this disorder. Neurobiol Aging. 1994;15:355-6.
- [CrossRef] [PubMed] [Google Scholar]
- Drugs acting on Central Nervous System In: Seth SD, ed. Text Book of pharmacology (2nd ed). New Delhi: Elsevier; 1999. p. :496-9.
- [Google Scholar]
- Prucalopride and donepezil act synergistically to reverse scopolamine-induced memory deficit in C57Bl/6j mice. Behav Brain Res. 2008;187:455-61.
- [CrossRef] [PubMed] [Google Scholar]
- Bioactive compounds and their derivatives: An insight into prospective phytotherapeutic approach against Alzheimer's disease. Oxid Med Cell Longev. 2022;2022:5100904.
- [CrossRef] [PubMed] [Google Scholar]
- In vitro anticancer activity of ethanolic extract of Cynodon dactylon against HT-29 cell line. Int J Curr Sci. 2013;5:74-81.
- [Google Scholar]
- Screening of antioxidant activity of three Indian medicinal plants, traditionally used for the management of neurodegenerative diseases. J Ethnopharmacol. 2003;84:131-8.
- [CrossRef] [PubMed] [Google Scholar]
- Role of aqueous extract of Cynodon dactylon in prevention of carbofuran-induced oxidative stress and acetylcholinesterase inhibition in rat brain. Cell Mol Biol (Noisy-le-grand). 2011;57:135-42.
- [Google Scholar]
- Secondary metabolites from Sida rhombifolia L. (Malvaceae) and the vasorelaxant activity of cryptolepinone. Molecules. 2013;18:2769-77.
- [CrossRef] [PubMed] [Google Scholar]
- A network pharmacology approach to reveal the underlying mechanisms of Paeonia lactiflora Pall. On the treatment of Alzheimer's disease. Evid Based Complement Alternat Med. 2019;2019:8706589.
- [CrossRef] [PubMed] [Google Scholar]
- α-Glucosidase inhibitors from Duranta repens modulate p53 signaling pathway in diabetes mellitus. Adv Tradit Med. 2020;20:427-38.
- [CrossRef] [Google Scholar]
- Gene set enrichment analysis of Alpha-glucosidase inhibitors from Ficus benghalensis. Asian Pac J Trop Biomed. 2019;9:263-70.
- [CrossRef] [Google Scholar]
- Elucidating type 2 diabetes mellitus risk factor by promoting lipid metabolism with gymnemagenin: An in vitro and in silico approach. Front Pharmacol. 2022;13:1074342.
- [CrossRef] [PubMed] [Google Scholar]
- Inhibitors of MAO-A and MAO-B in psychiatry and neurology. Front Pharmacol. 2016;7:340.
- [CrossRef] [PubMed] [Google Scholar]
- Apigenin ameliorates scopolamine-induced cognitive dysfunction and neuronal damage in mice. Molecules. 2021;26:5192.
- [CrossRef] [PubMed] [Google Scholar]
- Apigenin and related compounds stimulate adult neurogenesis. Mars, Inc., the Salk Institute for Biological Studies: WO2008147483. Expert Opin Ther Pat. 2009;19:523-7.
- [CrossRef] [PubMed] [Google Scholar]
- Neuroprotective effects of apigenin against inflammation, neuronal excitability and apoptosis in an induced pluripotent stem cell model of Alzheimer's disease. Sci Rep. 2016;6:31450.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of apigenin on tryptophan metabolic key enzymes expression in lipopolysaccharide-induced microglial cells and its mechanism. Heliyon. 2023;9:e12743.
- [CrossRef] [PubMed] [Google Scholar]
- Neuroprotective effects of quercetin in Alzheimer's disease. Biomolecules. 2019;10:59.
- [CrossRef] [PubMed] [Google Scholar]
- Protection against Alzheimer's disease by luteolin: Role of brain glucose regulation, anti-inflammatory activity, and the gut microbiota-liver-brain axis. Biofactors. 2021;47:218-31.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of luteolin on spatial memory, cell proliferation, and neuroblast differentiation in the hippocampal dentate gyrus in a scopolamine-induced amnesia model. Neurol Res. 2013;35:813-20.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of the most potent acetylcholinesterase inhibitors from plants for possible treatment of Alzheimer's disease: A computational approach. Egypt J Med Hum Genet. 2021;22:10.
- [CrossRef] [Google Scholar]
- Insight into antioxidant-like activity and computational exploration of identified bioactive compounds in Talinum triangulare (Jacq.) aqueous extract as potential cholinesterase inhibitors. BMC Complement Med Ther. 2024;24:134.
- [CrossRef] [PubMed] [Google Scholar]
- Serum expression of EAAT2 and ADORA2A in patients with different degrees of Alzheimer's disease. Eur Rev Med Pharmacol Sci. 2020;24:11783-92.
- [Google Scholar]
- 12/15-lipoxygenase is increased in Alzheimer's disease: Possible involvement in brain oxidative stress. Am J Pathol. 2004;164:1655-62.
- [CrossRef] [PubMed] [Google Scholar]
- Association between Alzheimer's disease and the NOS3 gene Glu298Asp polymorphism in Chinese. J Mol Neurosci. 2008;34:173-6.
- [CrossRef] [PubMed] [Google Scholar]
- Kaempferol attenuates cognitive deficit via regulating oxidative stress and neuroinflammation in an ovariectomized rat model of sporadic dementia. Neural Regen Res. 2018;13:1827-32.
- [CrossRef] [PubMed] [Google Scholar]
- Polyherbal and multimodal treatments: Kaempferol-and Quercetin-Rich herbs alleviate symptoms of Alzheimer's disease. Biology (Basel). 2023;12:1453.
- [CrossRef] [PubMed] [Google Scholar]
- Potential ameliorative effect of Cynodon dactylon (L.) pers on scopolamine-induced amnesia in rats: Restoration of cholinergic and antioxidant pathways. Indian J Pharmacol. 2021;53:50-9.
- [CrossRef] [PubMed] [Google Scholar]







