Translate this page into:
Rutin forestalls dysregulated cardiac bioenergetics in bisphenol A and dibutyl phthalate-exposed rats through PPARα and AMPK modulation
*Corresponding author: Olufemi Idowu Oluranti, Department of Physiology, Bowen University, Iwo, Osun State, Nigeria. olufemi.oluranti@bowen.edu.ng
-
Received: ,
Accepted: ,
How to cite this article: Oluranti OI, Alabi BA, Michael OS, Ojo AO, Akande AC. Rutin forestalls dysregulated cardiac bioenergetics in bisphenol A and dibutyl phthalate-exposed rats through PPARα and AMPK modulation. Indian J Physiol Pharmacol 2023;67:78-91.
Abstract
Objectives:
Proper cardiac function is greatly dependent on adequate supply and metabolism of energy substrates. Environmental pollutants exposure including plasticizers can trigger adverse cardiac metabolic events. This study was designed to investigate the ameliorative effect of rutin (Rt) on dysregulated cardiac energy metabolism in plasticizer-exposed rats.
Materials and Methods:
Forty-two rats were randomised into seven groups (n = 6): Control (0.1% dimethyl sulfoxide), bisphenol A (BPA, 25 mg/kg, p.o), dibutyl phthalate (DBP, 25 mg/kg, p.o), BPA + Rt 25 mg/kg, Rt 50 mg/kg, DBP + Rt (25 mg/kg, Rt 50 mg/kg), BPA + DBP and BPA + DBP + Rt, daily for 21 days.
Results:
BPA and DBP exposure increased plasma glucose, reduced insulin, and increased plasma and cardiac free fatty-acid. Cardiac glucose-6-phosphate level, hexokinase and pyruvate dehydrogenase activities increased in DBP while BPA reduced these variables. Cardiac glucose transporter-4 expression was reduced in BPA group, while cardiac peroxisome proliferator-activated receptor-alpha (PPARα) and AMP-activated protein kinase (AMPK) expression increased in BPA and DBP-treated rats. However, Rt administration prevents impaired cardiac bioenergetics and glucometabolic regulation.
Conclusion:
Summarily, Rt improves BPA and DBP-impaired cardiac bioenergetics through PPARα and AMPK modulation.
Keywords
Energy metabolism
Bisphenol A
Di-butyl phthalate
Rutin
Peroxisome proliferator-activated receptor-alpha (PPARα)
AMP-activated protein kinase
INTRODUCTION
The worldwide use of plastics has become so enormous to the extent that all aspect of daily life involves plastic. The excessive use of plastic products has raised serious concerns due to consistent exposure to plasticizers which could pose grave health risks to humans. Commonly used plasticizers are among the most pervasive environmental toxins in our environment and concern over their toxicity arose in the late 1990s, largely focused around undesirable reproductive and developmental effects. In recent times, studies have demonstrated that exposure to plasticizers increases the risk for obesity, type 2 diabetes (T2D) and cardiovascular disease.[1,2]
Bisphenol A (BPA) is the building block of plastics and Di-butyl phthalate (DBP) is a plasticizer used for flexibility and elasticity of plastic products.[3] They are important components of products such as intravenous bags, nylon water bags in West African countries, nasogastric tubes, umbilical catheters, enteral feeding tubes, haemodialysis tubing, endotracheal tubes, respiratory masks and examination gloves [3] These plastic products are hydrophobic and not covalently bound to plastic polymer, making them susceptible to leaching when they are in contact with water, plasma, blood and many other polar solvents in a container. Epidemiological and biomonitoring research has found a connection between exposure to BPA and DBP and a number of harmful health effects, including neurotoxicity, cardiotoxicity, haematotoxicity, nephrotoxicity and hepatitis.[4]
Furthermore, DBP and BPA were referred to as endocrine disruptors with a lot of reports on their carcinogenic potential, and developmental and reproductive toxicities.[5] Oxidative stress, dyslipidaemia, and myocardial injury, along with different stress pathways such as extracellular signal regulated kinase 1 and 2 (ERK1/2), Akt, p38 mitogen-activated protein kinase (MAPK) and nuclear factor-kappaB (NF-kB) have been linked with these plasticizers.[6] Oluranti et al.[7] also revealed that, oxidative stress, inflammatory mediators and activation of transcription factors like NFkB are major pathways of myocardial injury induced by DBP and BPA. Interestingly, from the pathophysiology of myocardial injury, abnormal shift in cardiac metabolism has been described to be a major cause of cardiomyopathy and other cardiac dysfunctions.[8] Liu et al.[9] showed a correlation between the disruption of mitochondrial metabolic process required for the generation of energy in form of adenosine triphosphate (ATP) and increase in the production of tissue inflammatory mediators, myocardial leucocyte infiltration and enhanced tissue level of reactive oxygen species (ROS) during cardiac injury. The disruption of cardiac metabolism and metabolic flexibilities during myocardial injury is known to affect glucose oxidation, fatty acid oxidation, serum level of triglyceride (TG) and free fatty acid (FFA), expression of enzymes involved in glycolytic pathways (pyruvate, pyruvate dehydrogenase [PDH], glucose-6-phosphate and hexokinase), cAMPK activity, GLUT-4 translocation and the activity of transcription factors such as peroxisome proliferator-activated receptor-alpha (PPAR-α).[10,11] Although, cardiac metabolic activities and flexibilities are disrupted during injury, there are differential regulations of cardiomyocyte enzyme and substrate during myocardial injury. The difference in the regulation of fatty acid and glucose metabolism depends on factors causing the injury, extent of ischemia and cardiomyopathy. Despite the study of Posnack et al.[12] that linked cardiotoxic effect of a phthalate with up-regulation of genes related to fatty acid transport, mitochondrial import and beta-oxidation, and esterification leading to an increase in myocyte fatty acid substrate utilisation, oxygen consumption, extracellular acidosis, PPAR-α expression and decrease in glucose oxidation, there is a paucity of scientific information on mechanisms by which DBP and BPA disrupt cardiac metabolism.
Polyphenols such as resveratrol, curcumin, apigenin and taxifolin were shown to protect the heart tissue against the cardiotoxic effects of phthalate through anti-oxidant, anti-inflammatory and anti-apoptotic responses in experimental rats.[13,14] Rutin (Rt) is a flavonoid that can be derived from a variety of natural sources, including oranges, buckwheat, grapes, peaches, lemons, limes and berries, to name a few.[15] Despite the anti-inflammatory, antioxidant and anti-apoptotic role of these flavonoids, it is not clear whether they could reverse the disrupted cardiac metabolism associated with plasticizer-induced myocardial injury. Hence, we designed this study to evaluate the protective role of Rt against the disruption of cardiac metabolism in DBP and BPA exposure.
MATERIALS AND METHODS
Animals
Following the requirements of the National Institutes of Health Guide for the Treatment and Use of Laboratory Animals, the Bowen University Ethical Review Committee approved the experimental guidelines with the reference number BUREC/07i/20 on 23rd February, 2021. The Central Animal House, College of Health Sciences, Bowen University, Iwo, Nigeria, provided 42 male Wistar rats with body weights of about 150 g. The rats were fed regular rat food and given free access to water on a daily basis, with a 12 h light/dark cycle.
Experimental design
After a week of acclimatisation, all 42 rats were divided into seven groups, each with six animals, and treated orally for 21 days as follows: Control (0.1% dimethyl sulfoxide), BPA, 25 mg/kg, p.o, DBP, 25 mg/kg, p.o, BPA + Rt (25 mg/kg, 50 mg/kg), DBP + Rt, BPA +DBP, BPA + DBP + Rt. BPA, DBP and Rt. The doses were in accordance with the previous research.[7]
Sample collection
The retro-orbital complex was used to collect blood samples from the rats after an overnight fast. The blood was centrifuged at 3000 rpm for 10 min (rpm). For further examination, plasma was extracted and kept at 20°C. Animals were sacrificed by cervical dislocation under mild anaesthesia and opened up by abdomen-pelvic incision to remove the heart, cleansed of adherent connective tissues, blotted and weighed. The hearts were homogenised in 4 times phosphate buffer weight/volume (pH 7.4), centrifuged for 15 min at 10,000 rpm and the supernatant fraction was used for biochemical analysis. For immunohistochemical staining, a piece of cardiac tissue was fixed in phosphate buffered formaldehyde (10%).
Biochemical analysis
Plasma insulin and glucose
The amount of plasma glucose was calculated using the Trinder technique and a commercial kit (Sigma Diagnostics Pvt. Ltd., Baroda, India).[16] Plasma insulin was measured using an enzyme-linked immunosorbent assay kit (Boehringer-Mannheim Kit, Mannheim, Germany).
Lipid profile
The levels of TGs and FFA in plasma and cardiac tissue homogenates were determined using standardised colorimetric procedures and reagents from Fortress Diagnostics Ltd. (Antrim, UK).
Tissue glycogen
For this assay, 100 mg of heart tissue was alkali digested (5 mL of 30% w/v Potassium hydroxide) in a boiling water tank for 20 min and then cooled to room temperature. A drop in ammonium acetate was followed by 3.0 mL of pure ethanol. The resultant mixture was centrifuged at 3000 g for 10 min to collect the precipitate. The precipitated glycogen sample aliquot was cooked for 20 min in a boiling water bath with 4 ccs of anthrone reagent. At 640 nm, the concentration of myocardial glycogen (green colour) was determined and expressed in moist tissue mg/g.[17]
Tissue pyruvate and glucose-6-phosphate
Using Fortress Diagnostics Ltd. assay kits, tissue levels of pyruvate and glucose-6-phosphate were quantified using a standardised colorimetric technique (Antrim, UK).
Hexokinase
The activity of hexokinase, a glucose metabolism enzyme, was measured. 5.3 mL glucose (1 mL, 5 mM), ATP (0.5 mL, 4 mM), potassium chloride (0.4 mL, 7 mM), potassium dihydrogen phosphate (0.4 mL), magnesium chloride (0.1 mL, 100 mM), sodium fluoride (0.4 mL) and 50 mM Tris-hydrochloric acid buffer (2.5 mL, pH8.0). The reaction mixture was pre-incubated at 370°C for 5 min before adding cardiac homogenate (2 mL) to start the reaction. A volume of 1 mL was immediately transferred to the trichloroacetic acid-containing tubes (10% w/v, 1 mL) and incubated for 30 min at 370°C. Following centrifugation, the remaining glucose content in the supernatant was quantified at 340 nm to determine hexokinase activity.
PDH
The activities of PDH were determined using the spectrophotometric method of Szutowicz et al.[18]
Immunohistochemistry staining
Immunohistochemistry was used to assess the protein expression of glucose transporter 4 (GLUT4), CPT1, PPAR and AMP-activated protein kinase (AMPK) in cardiac tissue, as described by Alabi et al.[19] The cardiac tissues were maintained with 10% formaldehyde and sliced into 5-m thick sections on poly-lysine-coated glass slides. The sections were boiled in 10 mM citrate buffer (pH 6.5) for 20 min to extract the antigens. To reduce non-specific staining, sections were treated in hydrogen peroxide for 15 min before being washed 3 times (5 min each time) in ×1 PBST (0.05% Tween 20). After applying blocking solution for 10 min, slices were incubated with bioss diluted primary antibodies against GLUT4, CPT1, PPAR and AMPK. Anti-GLUT4 (1:200), anti-CPT1 (1:100), anti-PPAR (1:200) and anti-AMPK (1:100) antibodies were incubated at 4°C overnight. The Ultra Vision Plus Detection System Anti-Polyvalent, HRP/3, 3'-diaminobenzidine (DAB) (Ready-To-Use) staining kit from Thermo Scientific, was used for further processing. DAB was used to visualize the peroxidase complex. Finally, the slides were counterstained with haematoxylin, washed in xylene, and dehydrated in ethanol and DPX mounted microscopic analysis (Olympus BX-51) was performed. The Image J software program from Nottingham was used to do a quantitative study of protein expression from the photomicrograph taken using the total area of protein expression.
Data analysis
All data were presented as means with standard error of mean (SEM). GraphPad prism 5 statistical software was used to conduct statistical analysis. To compare the mean values of variables among the groups, one-way analysis of variance (ANOVA) was employed. The significance of pairwise comparisons of mean values among the groups was determined with a post hoc test (Newman Keul’s test). At P ≤ 0.05, mean differences were considered statistically significant.
RESULTS
[Figure 1] Effects of rutin on (a) fasting blood glucose (b) fasting insulin, in plasticizers exposed Wistar rats.
![Effects of Rt on (a) fasting blood glucose and (b) fasting insulin, in plasticizers exposed Wistar rats. The fasting blood glucose increased (P < 0.05 [P = 0.0312]) following administration of BPA and DBP while the introduction of Rt reduced the fasting blood glucose level (P = 0.0249). The fasting insulin decreased significantly in BPA + Rt when compared to BPA (P = 0.0270) while it increased significantly in DBP + Rt and BPA +DBP + Rt when compared with DBP (P = 0.0225) and BPA + DBP (P = 0.0493), respectively. Data are expressed as mean ± SEM. n = 6 and analysed by one-way ANOVA, followed by Newman–Keuls post hoc test. (*P < 0.05 vs. control; #P < 0.05 vs. BPA + DBP; aP < 0.05 vs. BPA; bP < 0.05 vs. DBP). BPA: Bisphenol A, DBP: Dibutyl phthalate, Rt: Rutin, SEM: Standard error of mean, n: number.](/content/114/2023/67/2/img/IJPP-67-078-g001.png)
- Effects of Rt on (a) fasting blood glucose and (b) fasting insulin, in plasticizers exposed Wistar rats. The fasting blood glucose increased (P < 0.05 [P = 0.0312]) following administration of BPA and DBP while the introduction of Rt reduced the fasting blood glucose level (P = 0.0249). The fasting insulin decreased significantly in BPA + Rt when compared to BPA (P = 0.0270) while it increased significantly in DBP + Rt and BPA +DBP + Rt when compared with DBP (P = 0.0225) and BPA + DBP (P = 0.0493), respectively. Data are expressed as mean ± SEM. n = 6 and analysed by one-way ANOVA, followed by Newman–Keuls post hoc test. (*P < 0.05 vs. control; #P < 0.05 vs. BPA + DBP; aP < 0.05 vs. BPA; bP < 0.05 vs. DBP). BPA: Bisphenol A, DBP: Dibutyl phthalate, Rt: Rutin, SEM: Standard error of mean, n: number.
[Figure 2] Effects of rutin on plasma and cardiac (a&b) TG and (c&d) FFA in plasticizers exposed Wistar rats.
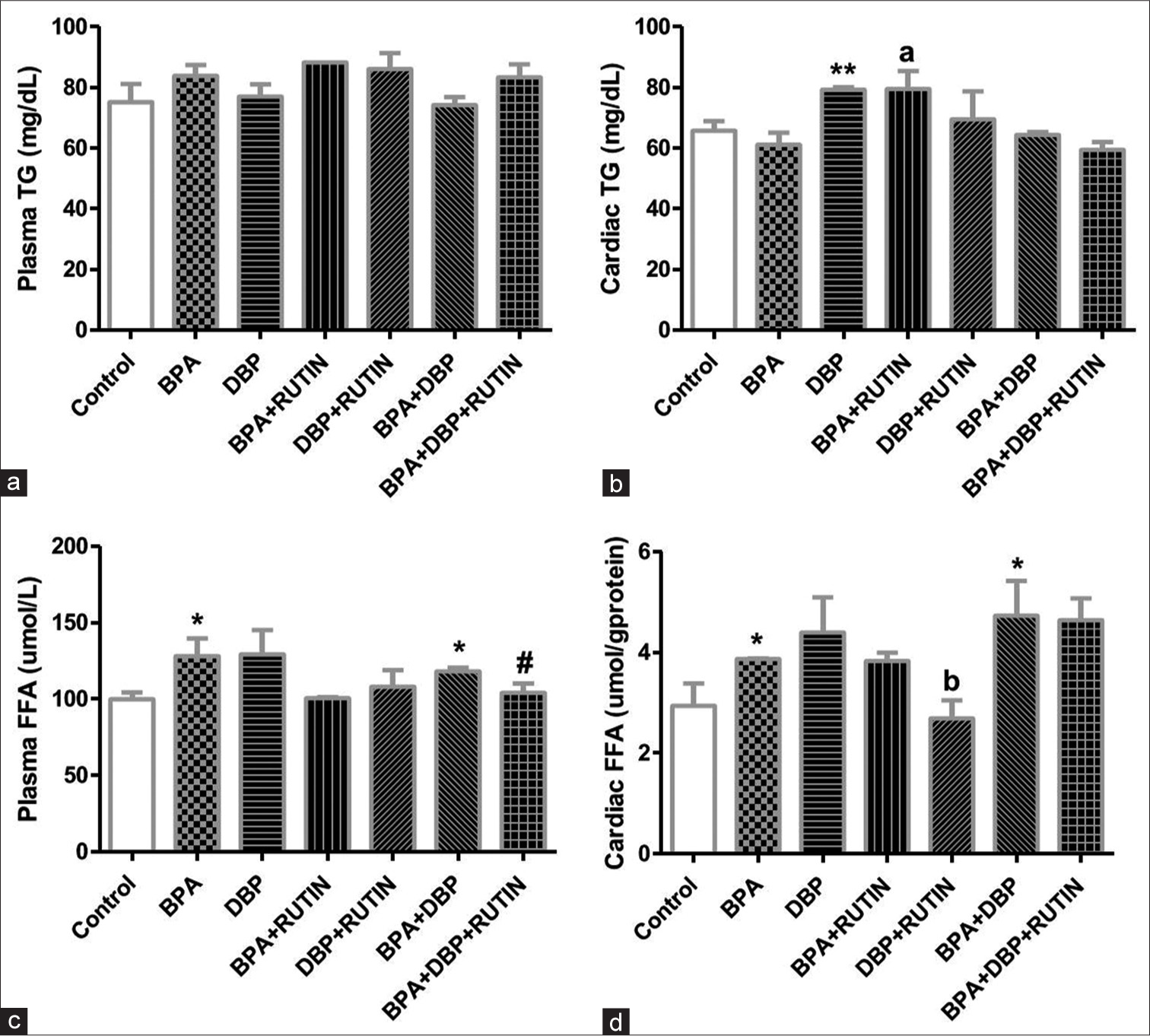
- Effects of Rt on plasma and cardiac (a and b) TG and (c and d) FFA in plasticizers exposed Wistar rats. There were no significant changes in plasma TG, the cardiac TG increased significantly following DBP administration when compared with control (P = 0.0074) as the level increased in BPA + Rt when compared to BPA (P = 0.0297). The plasma FFA increased in BPA and BPA + DBP when compared to control (P = 0.0439, 0.0118) and this was reduced following the administration of Rt in BPA + DBP + Rt group (P = 0.0506). The cardiac FFA significantly increased in BPA and BPA + DBP when compared to control (P = 0.0518, 0.0471) while a significant reduction was observed when Rt was administered with DBP when compared to only DBP (P = 0.0481). Data are expressed as mean ± SEM. n = 6 and analysed by one-way ANOVA, followed by Newman–Keuls post hoc test. (*P < 0.05 vs. control; #P < 0.05 vs. BPA + DBP; bP < 0.05 vs. DBP). Rt: Rutin, BPA: Bisphenol A, DBP: Dibutyl phthalate, TG: Triglyceride, FFA: free fatty acids.
[Figure 3] Effects of rutin on cardiac (a) glycogen (b) hexokinase (c) pyruvate dehydrogenase (d) glucose 6 phospahte (e) pyruvate, in plasticizers exposed Wistar rats.
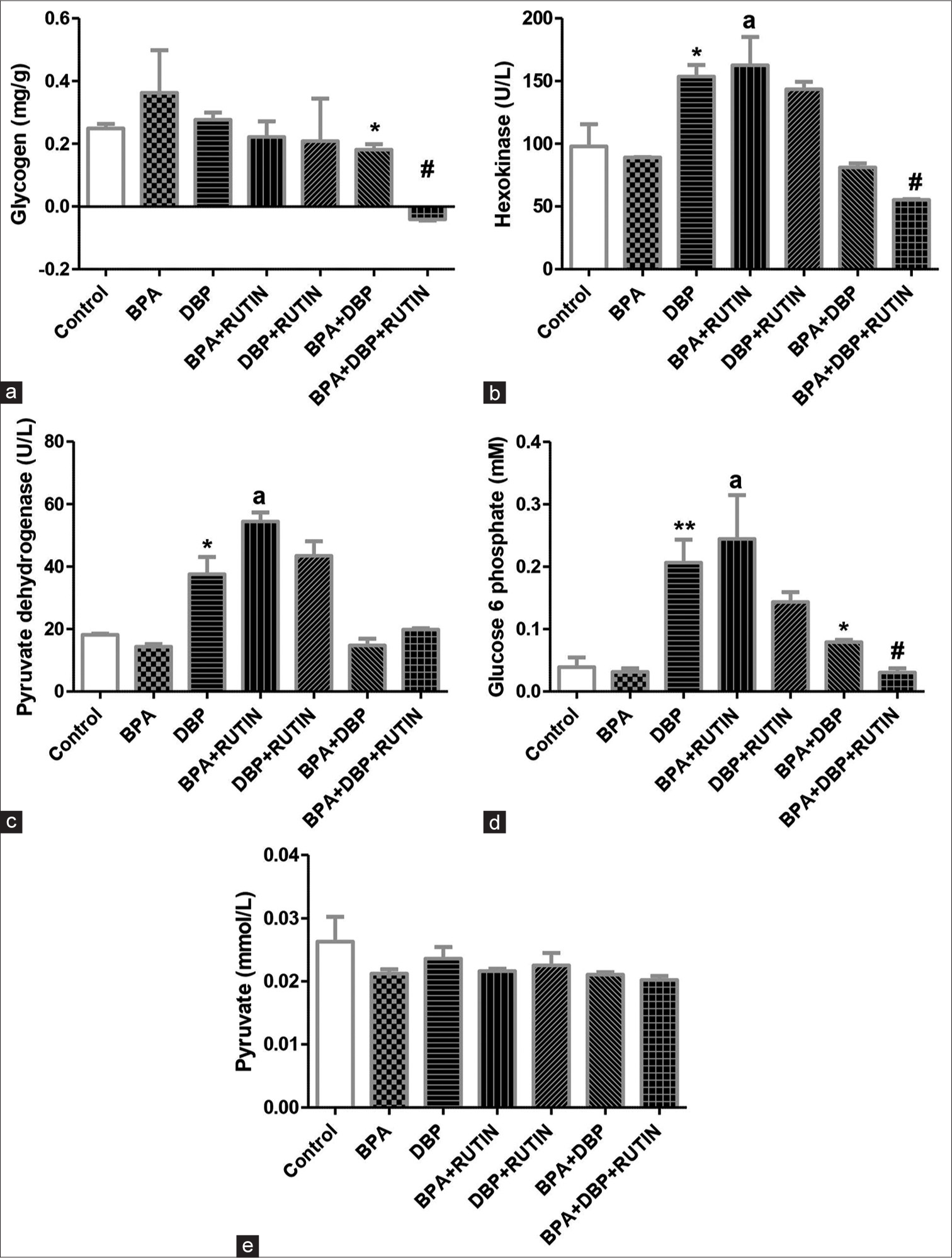
- Effects of Rt on cardiac (a) glycogen, (b) hexokinase, (c) pyruvate dehydrogenase, (d) glucose 6 phosphate and (e) pyruvate, in plasticizers exposed Wistar rats. The glycogen level was reduced in BPA + DBP when compared with control (P = 0.0477) and reduced further with administration of Rt as seen in BPA + DBP + Rt group (p = 0.0035). There was an increase in hexokinase and pyruvate dehydrogenase activities in DBP and BPA + Rt when compared to control (P = 0.0542, 0.0359) and BPA (P = 0.0410, 0.0029), respectively. Glucose 6 phosphate levels increased in DBP and BPA + Rt when compared to control (P = 0.0072) and BPA (P = 0.0196), respectively; it also increased in BPA + DBP when compared to the control (P = 0.0336); however, the introduction of Rt reduced it significantly (P = 0.0016). The pyruvate level remains unchanged. Data are expressed as mean ± SEM. n = 6 and analysed by one-way ANOVA, followed by Newman–Keuls post hoc test. (*P < 0.05, **P < 0.01 vs. control; #P < 0.05 vs. BPA + DBP; aP < 0.05 vs. BPA). Rt: Rutin, BPA: Bisphenol A, DBP: Dibutyl phthalate.
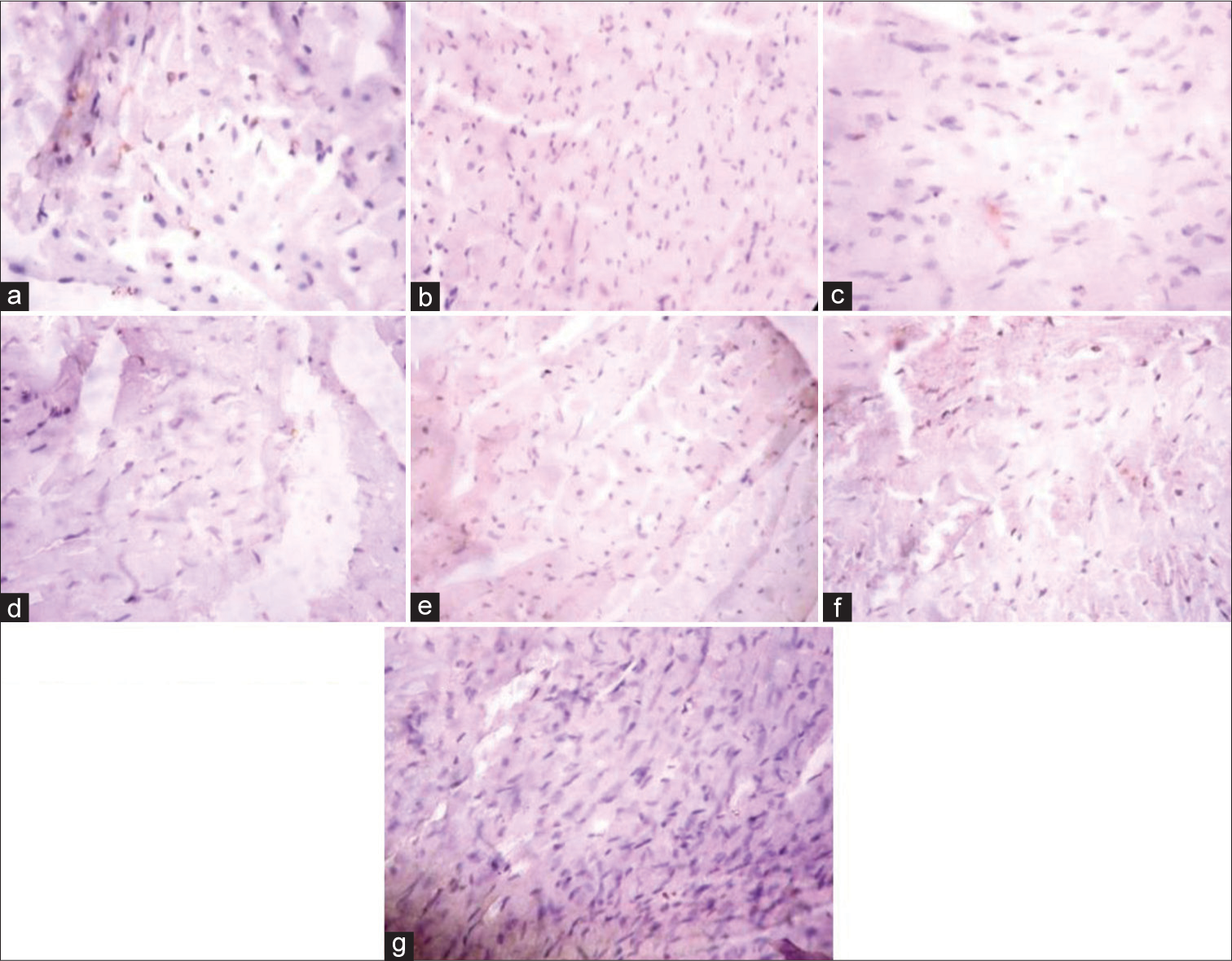
[Figure 4] (Plate a-g): Photomicrograph of a heart section stained immunohistochemically for GLUT 4 expression (X400). a (Control), b (BPA), c (DBP), d (BPA + Rutin), e (DBP + Rutin), f (BPA +DBP), g (BPA +DBP + Rutin).
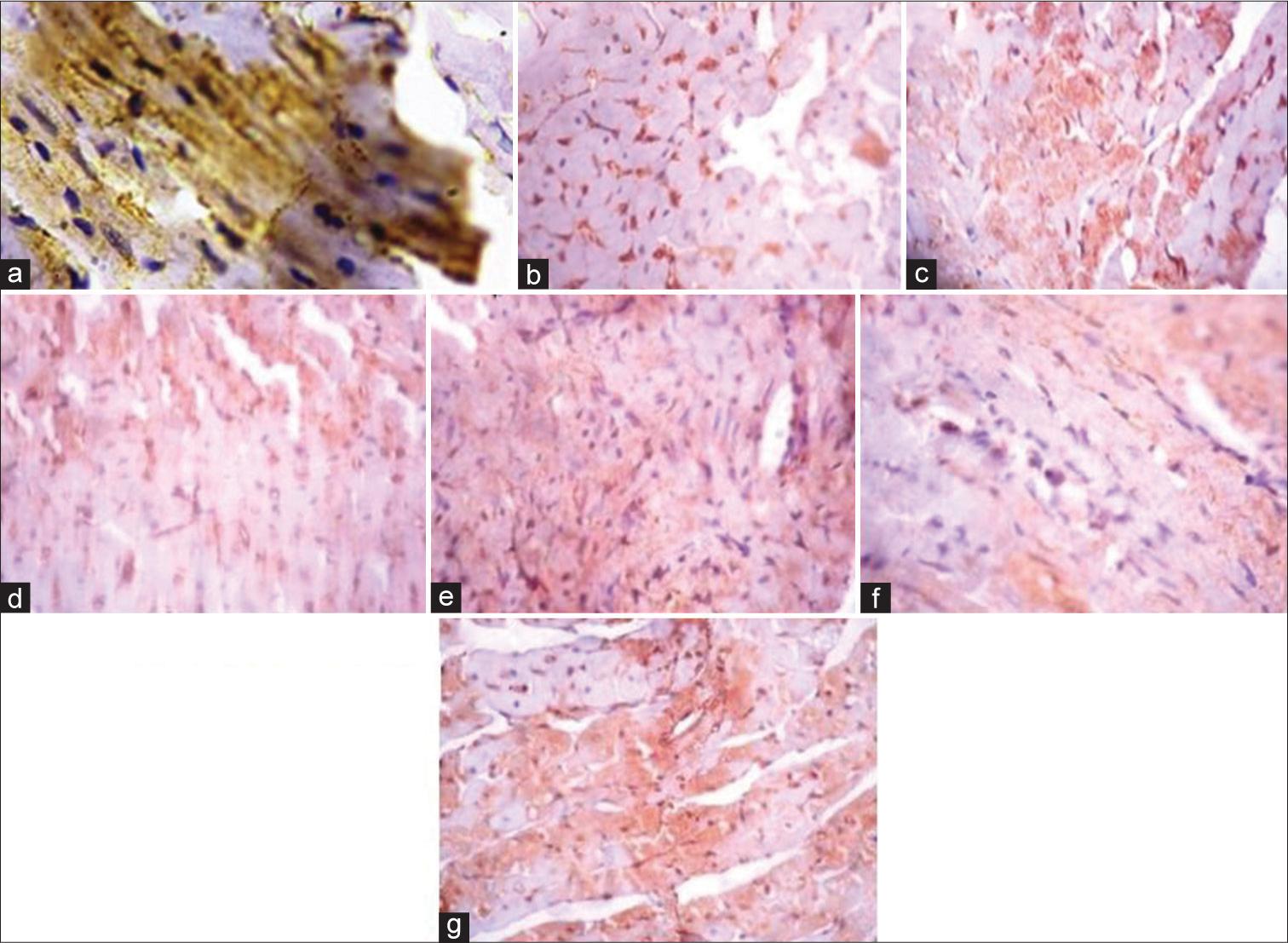
- (Plate a-g): Photomicrograph of a heart section stained immunohistochemically for GLUT 4 expression (×400). (a) (Control), (b) (BPA), (c) (DBP), (d) (BPA + Rt), (e) (DBP + Rt), (f) (BPA + DBP) and (g) (BPA + DBP + Rt). Rt: Rutin, BPA: Bisphenol A, DBP: Dibutyl phthalate.
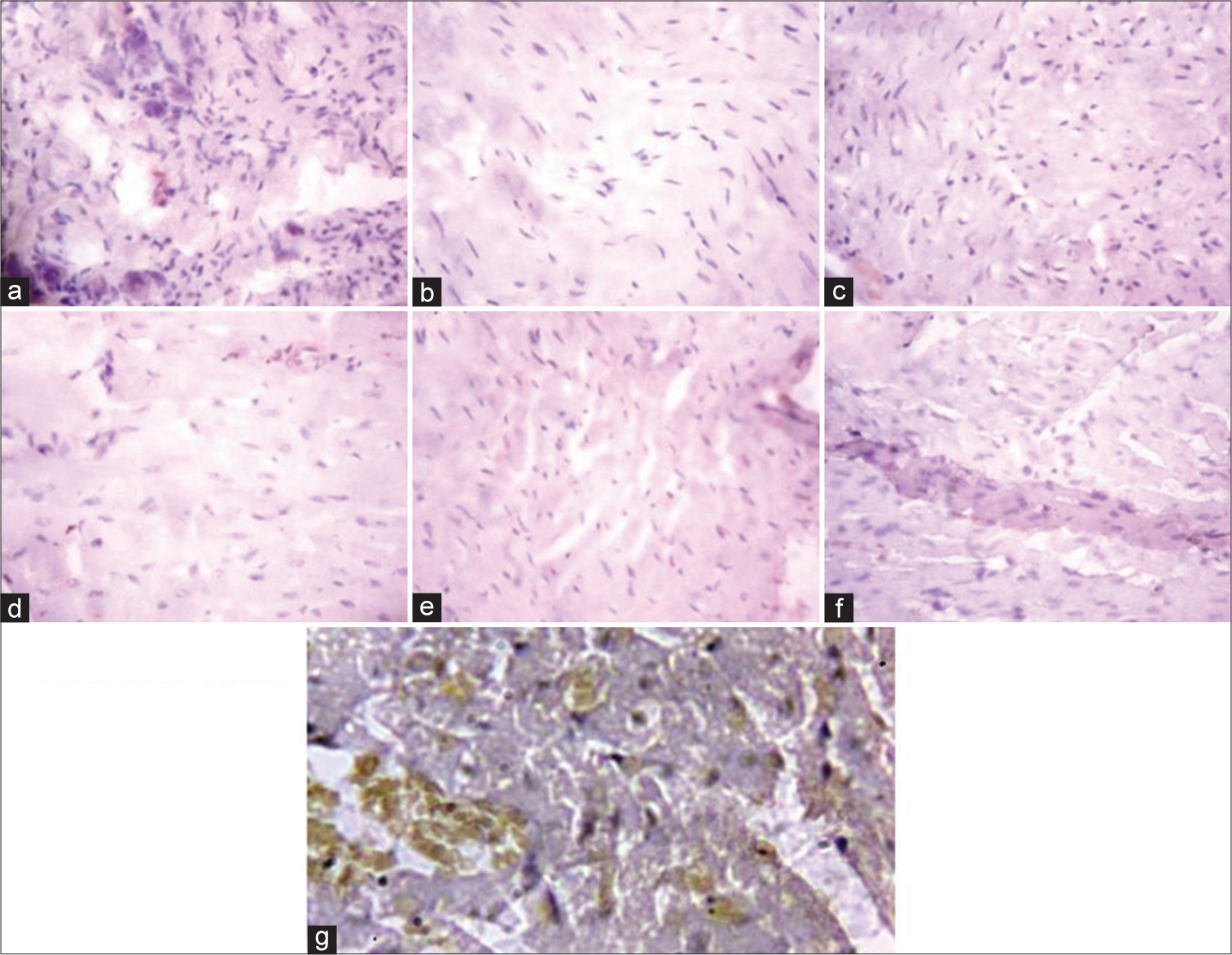
[Figure 5] (Plate a-g): Photomicrograph of a heart section stained immunohistochemically for CPT1β expression (X400). a (Control), b (BPA), c (DBP), d (BPA + Rutin), e (DBP + Rutin), f (BPA +DBP), g (BPA +DBP + Rutin).
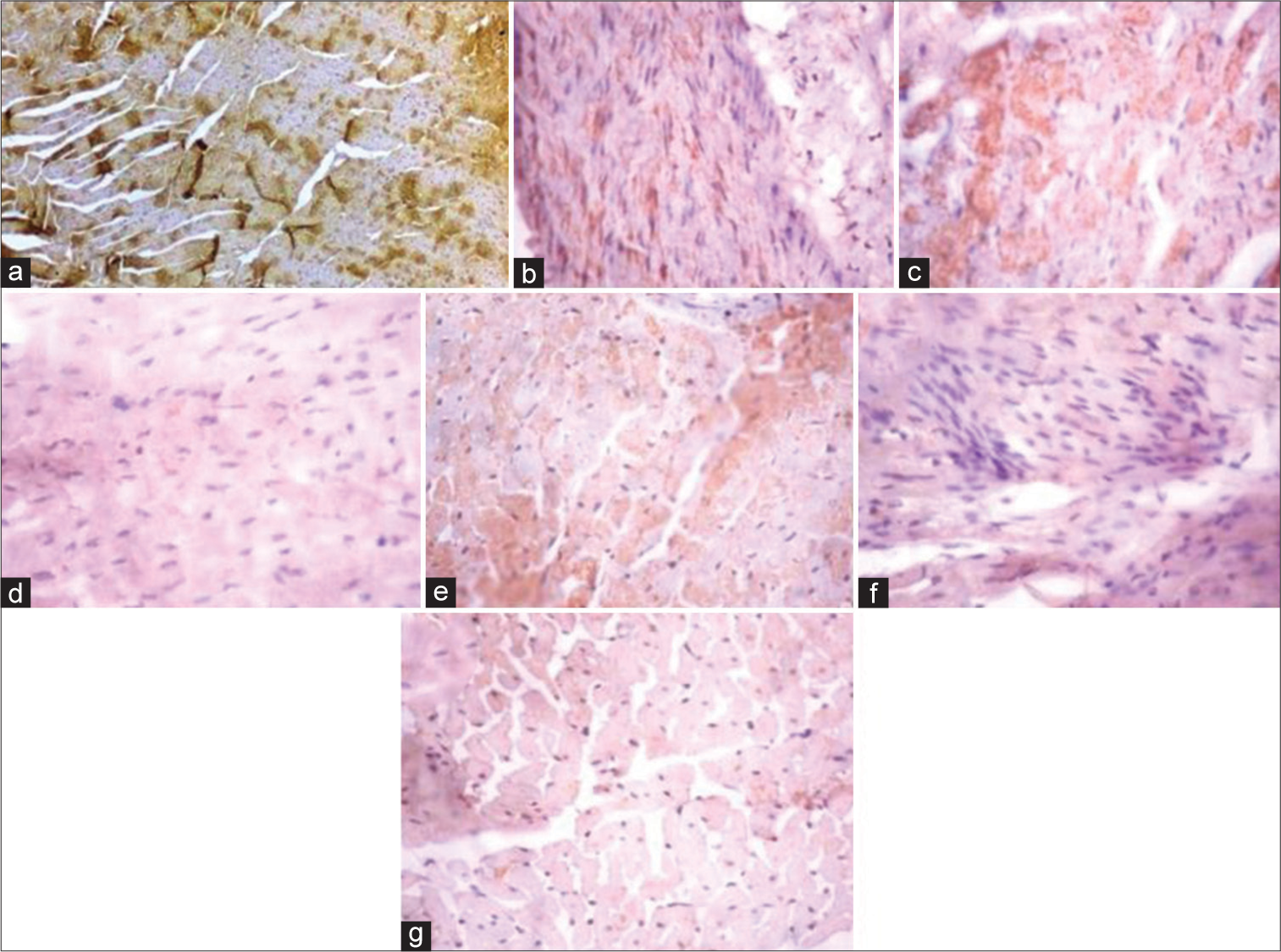
- (Plate a-g): Photomicrograph of a heart section stained immunohistochemically for carnitine palmitoyltransferase 1β expression (×400). (a) (Control), (b) (BPA), (c) (DBP), (d) (BPA + Rt), (e) (DBP + Rt), (f) (BPA + DBP) and (g) (BPA + DBP + Rt). BPA: Bisphenol A, DBP: Dibutyl phthalate, Rt: Rutin.
[Figure 6] Effects of rutin on cardiac (a) GLUT4 (b) CPT1β, in plasticizers exposed Wistar rats.
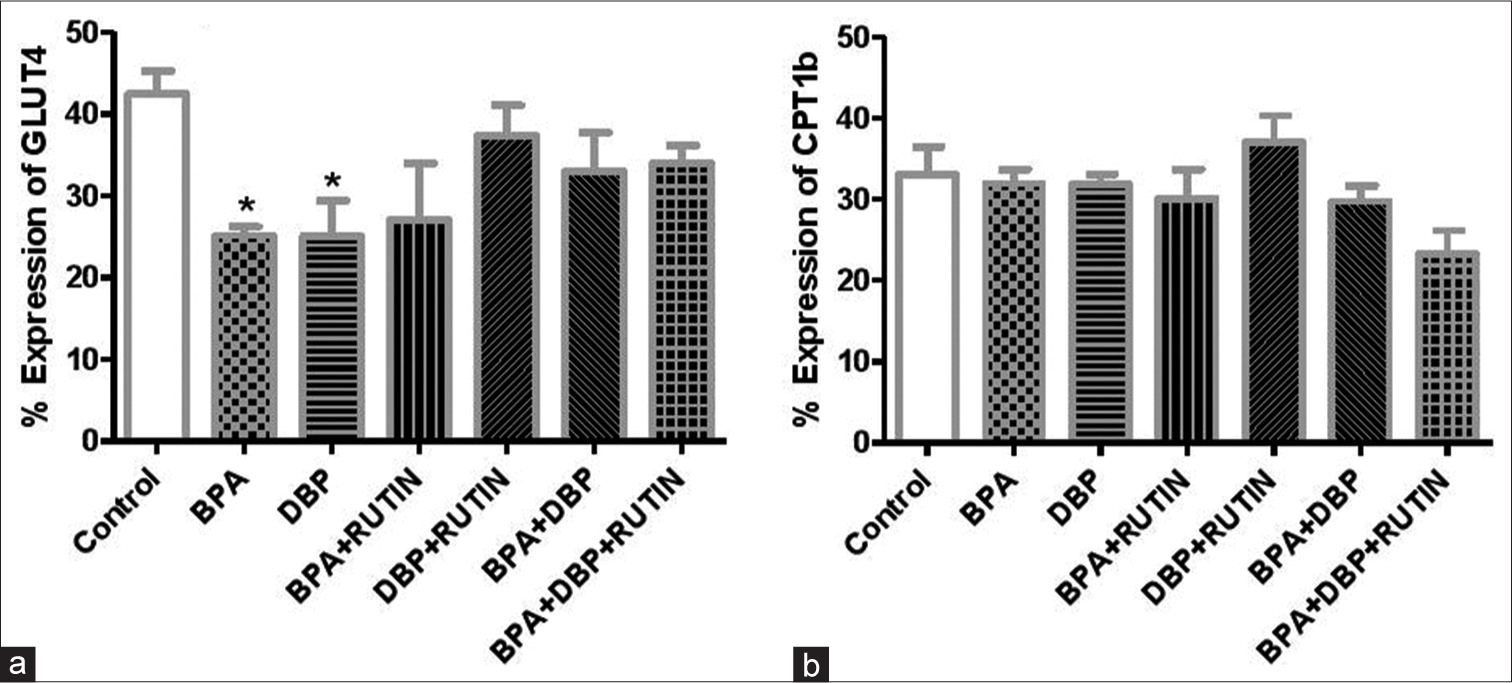
- Effects of Rt on cardiac (a) GLUT4 and (b) carnitine palmitoyltransferase 1β, in plasticizers exposed Wistar rats. Effects of Rt on cardiac (a) GLUT4 and (b) CPT1β, in plasticizers exposed Wistar rats. The % expression of GLUT 4 decreased significantly (P < 0.05) following administration of BPA and DBP when compared to the control (P = 0.0145, 0.0394). There were no significant changes in the % expression of CPT1β. Data are expressed as mean ± SEM. n = 6 and analysed by one-way ANOVA, followed by Newman–Keuls post hoc test (*P < 0.05 vs. control). Rt: Rutin, BPA: Bisphenol A, DBP: Dibutyl phthalate, GLUT4: Glucose transporter 4, CPT1β: Carnitine palmitoyltransferase 1β.
[Figure 7] (Plate a-g): Photomicrograph of a heart section stained immunohistochemically for PPARα expression (X400). a (Control), b (BPA), c (DBP), d (BPA + Rutin), e (DBP + Rutin), f (BPA +DBP), g (BPA +DBP + Rutin).
- (Plate a-g): Photomicrograph of a heart section stained immunohistochemically for PPARα expression (× 400). (a) (Control), (b) (BPA), (c) (DBP), (d) (BPA + Rt), (e) (DBP + Rt), (f) (BPA + DBP) and (g) (BPA + DBP +Rt). BPA: Bisphenol A, DBP: Dibutyl phthalate, Rt: Rutin.
[Figure 8] (Plate a-g): Photomicrograph of a heart section stained immunohistochemically for AMPK expression (X400). a (Control), b (BPA), c (DBP), d (BPA + Rutin), e (DBP + Rutin), f (BPA +DBP), g (BPA +DBP + Rutin).
- (Plate a-g): Photomicrograph of a heart section stained immunohistochemically for activated protein kinase expression (× 400). (a) (Control), (b) (BPA), (c) (DBP), (d) (BPA + Rt), (e) (DBP + Rt), (f) (BPA + DBP) and (g) (BPA + DBP + Rt). BPA: Bisphenol A, DBP: Dibutyl phthalate, Rt: Rutin.
[Figure 9] Effects of rutin on cardiac (a) PPARα (b)AMPK, in plasticizers exposed Wistar rats.
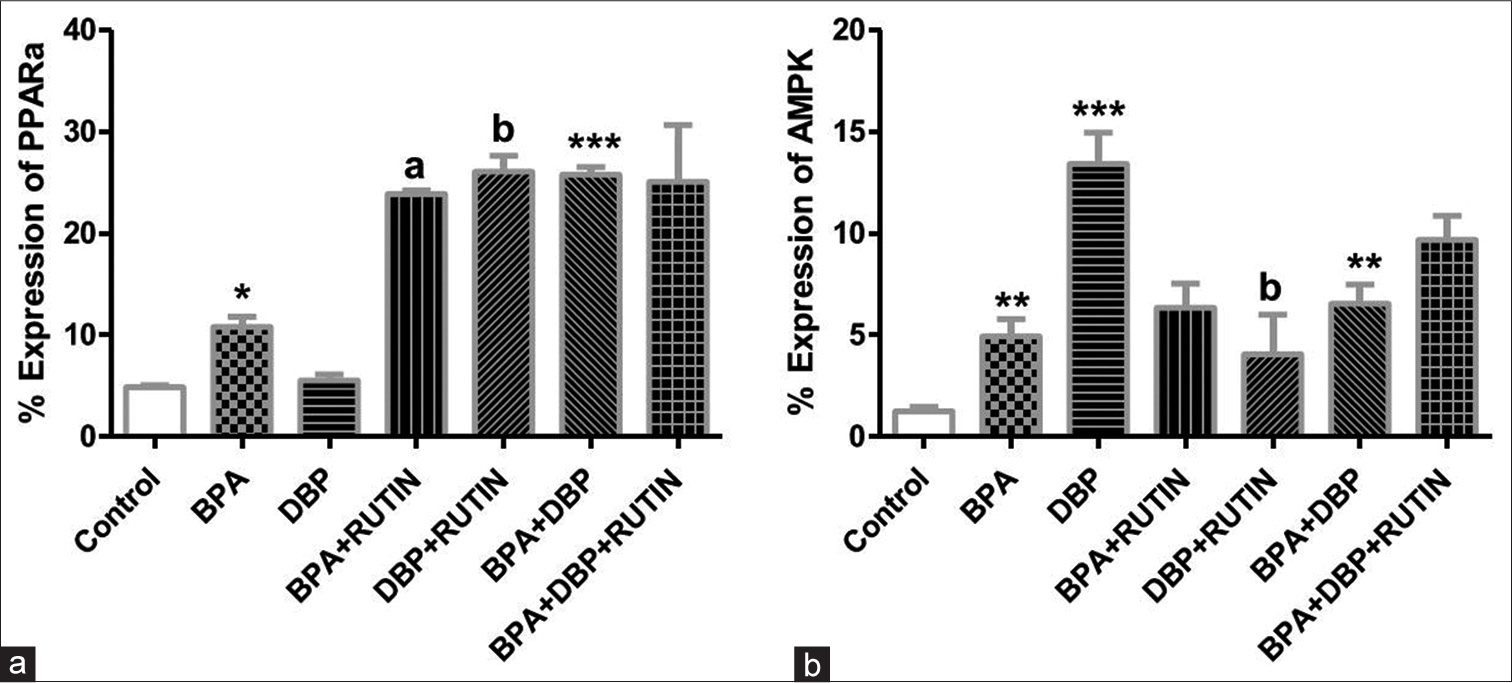
- Effects of Rt on cardiac (a) PPARα and (b) AMPK, in plasticizers exposed Wistar rats. Effects of Rt on cardiac (a) PPARα and (b) AMPK, in plasticizers exposed Wistar rats. The % expression of PPARα increased significantly in BPA and BPA + DBP when compared to the control (P = 0.0140, 0.0007) while it reduced significantly in BPA + Rt and DBP + Rt compared with BPA and DBP groups (P = 0.0032, 0.0031). There was an increase in AMPK expression following administration of the plasticizers as seen in BPA, DBP and BPA + DBP groups compared with control (P = 0.0070, 0.0010, 0.0028) while the administration of Rt reduced the expression in DBP + Rt when compared with DBP only (P = 0.0318). Data are expressed as mean ± SEM. n = 6 and analysed by one-way ANOVA, followed by Newman–Keuls post hoc test.(*P < 0.05, **P < 0.01, ***P < 0.001 vs. control; aP < 0.05 vs. BPA; bP < 0.05 vs. DBP). BPA: Bisphenol A, DBP: Dibutyl phthalate, Rt: Rutin, PPARα: Peroxisome proliferator activated receptor alpha, AMPK: Adenosine monophosphate kinase.
[Figure 10] Schematic pathway illustrating the likely pathway via which rutin protect the cardiac tissue from energy inefficiency induced by bisphenol-A and dibutyl phthalate.
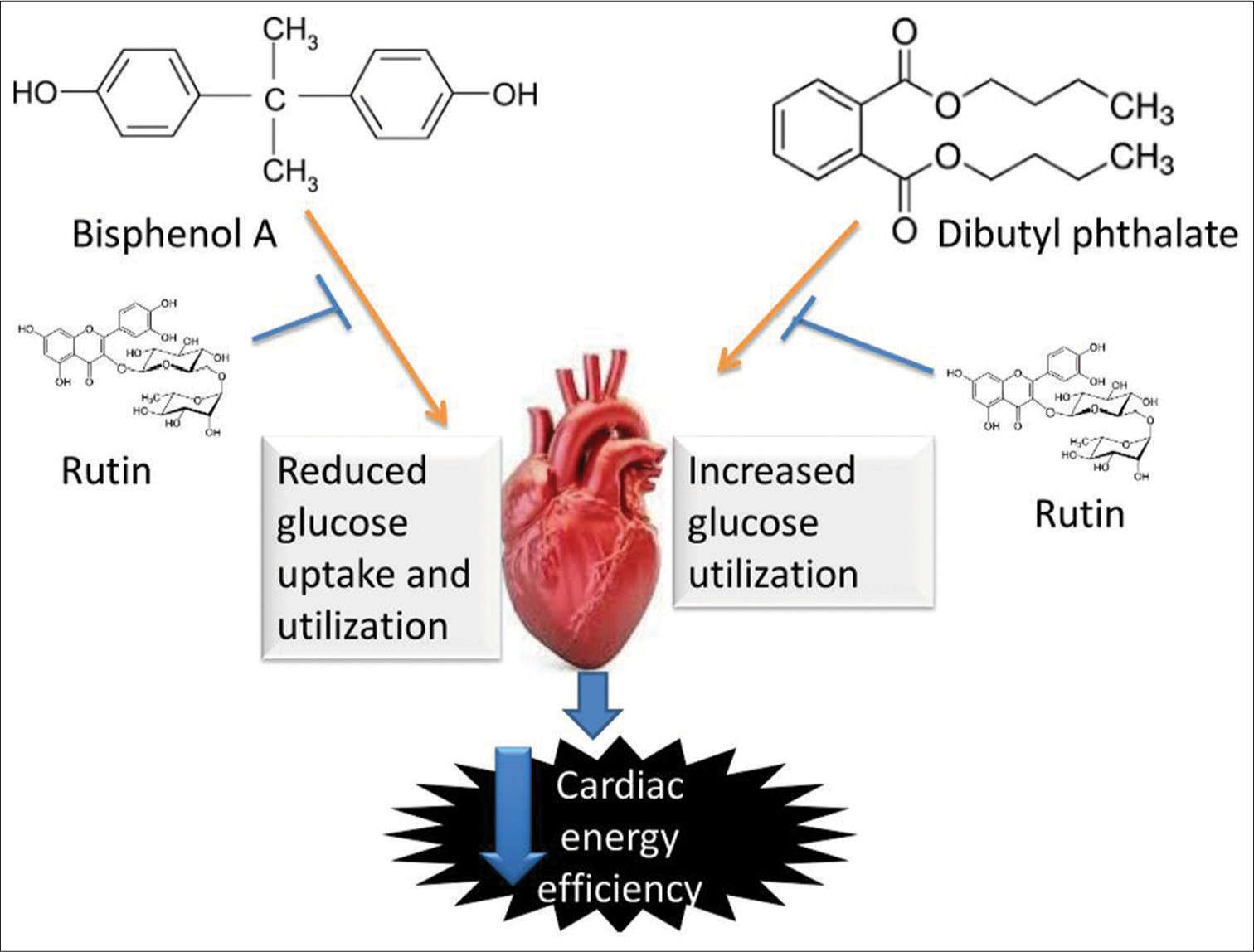
- Schematic pathway illustrating the likely pathway through which rutin protect the cardiac tissue from energy inefficiency induced by bisphenol A and dibutyl phthalate.
DISCUSSION
The heart is an extremely active metabolic tissue demanding huge and constant supply of energy for its activities. Hence, regular energy generation in the form of ATP from metabolic substrates provides the fuel the cardiomyocytes need for its metabolic and normal contractile functions.[20] The previous investigators have reported the deleterious impacts of either singly or combine administration of BPA and DBP on cardiac and metabolic health.[1,21] Hence, the present study was designed to investigate the ameliorative role of Rt on cardiac dysmetabolism induced by DBP and BPA, through the regulation of AMPK and PPARα in the myocardial energy homeostasis.
The finding of this study revealed that coadministration of BPA and DBP increased the blood glucose level (P < 0.05) corroborating earlier studies. Kwack et al. investigated the short-term effects of phthalate monoesters and diesters on male rats, finding that glucose levels were considerably higher in the Di-(2-ethylhexyl) phthalate (DEHP) groups than in the control group.[22] DEHP and its metabolites have been linked to elevated blood glucose levels and insulin resistance in the past.[23] In adult female Wistar rats, DEHP-exposed pups had higher blood glucose levels, lower serum insulin levels, and reduced glucose tolerance and insulin secretion.[23] In a previous study, rats exposed to bisphenol had significantly higher blood glucose levels than control rats,[24] indicating a disruption in glucose metabolism homeostasis.
In the present investigation, BPA and coadministration of BPA and DBP increased the plasma and cardiac FFA (P < 0.05). In agreement with our observation, Abdel-Rahman et al.[25] and Mahmoudi et al.[26] both came to similar conclusions. It is possible that BPA’s potential to impair lipid metabolism and cause lipid accumulation is due to the development of 3T3-L1 fibroblasts into adipocytes.[27] BPA has been linked to changes in human lipid profiles and obesity, which can lead to insulin resistance and/or diabetes mellitus.[28] BPA treatment (0.5g of BPA/kg/day) resulted in considerable build-up of TGs and cholesterol in the liver of male mice from birth to 10 months.[29] In experimental rats, BPA exposure elevated serum levels of TGs and FFAs.[30] Li et al. found that obese type 2 diabetes mellitus mice had significant cardiac lipotoxicity, which was marked by increased FFA absorption and lipid droplet formation.[31] Under physiological conditions, FFAs are essential sources of energy in the body, and cardiomyocytes get 60–70% of their energy through full oxidation of FFAs.[31] Plasma FFAs, on the other hand, rise dramatically in obesity, metabolic syndrome, diabetes and other pathological situations. Excess FFAs accumulate in non-fat cells, causing glucose and lipid metabolic problems such as lipotoxicity and insulin resistance.[32] Excess FFAs can also cause an imbalance in FFA uptake and utilisation in cardiomyocytes, leading to excessive FFA absorption and oxidation in the heart, which can lead to cardiomyocyte injury.[33]
Hexokinase is the first glycolytic enzyme to phosphorylate glucose, which is the first and rate-limiting step in the glycolytic process, by transferring the phosphoryl group from ATP to glucose-6-phosphate.[34] The rate-limiting enzyme for glucose oxidation is PDH, which regulates the flow of pyruvate into the mitochondria for oxidative metabolism.[35] In this study, BPA reduced the activities of hexokinase and PDH by 10% and 26%, respectively, while DBP increased the activities significantly (P < 0.05) compared with control. Glucose-6-phosphate level was reduced in BPA group by 25% and increased significantly (P < 0.05) in DBP group. These findings show that glucose metabolism has been suppressed in BPA exposed animals which are typical of a diabetic heart while it increases in DBP exposed group which is typical of cardiac hypertrophy. One of the most well-known endocrine disrupting chemicals is BPA. There is a substantial link between BPA exposure and the development of metabolic illnesses like obesity and T2D, according to epidemiological and clinical research.[36,37] In the hearts of db/db mice, the rate-limiting enzymes of glucose oxidation, including PDH, reduced when compared to control group.[38] Evidence suggests that the diabetic heart undergoes substrate preference modification. In diabetic cardiomyocytes, impaired glucose uptake and reduced glucose oxidation are prevalent, along with a shift toward increasing reliance on fatty acid oxidation.[39] Higher fatty acid oxidation (due to increased fatty acid absorption) may also reduce glucose oxidation through the Randle cycle.[40] In some diabetes-modelling rodents, the capacity for pyruvate oxidation was shown to be reduced.[41] Lower glucose oxidation is evident by 4 weeks of age in hereditary mice models of obesity (ob/ob) and T2D (db/db), which is associated with an increase in fatty acid oxidation and reduced heart efficiency, and precedes hyperglycaemia.[42] Our findings backed with previous observations that DEHP exposure causes an increase in glucose level, which is one of the direct causes of heart hypertrophy.[43] Excess glucose intake, according to evidence,[44] exacerbates the development of ventricular hypertrophy due to aberrant glucose metabolism, as seen in this study with elevated glucose metabolic enzyme activities.
The % expression of cardiac GLUT 4 decreased significantly (P < 0.05) following administration of BPA and DBP when compared to the control. There were no significant changes in the % expression of cardiac carnitine palmitoyltransferase 1β. GLUT4 is the most common glucose receptor in adult hearts, and it is responsible for the majority of glucose transfer into cardiomyocytes.[45] Glycometabolism disorders have been linked to an increase in glucose intake and glycolysis in patients with pathological heart hypertrophy, according to other research.[46] DEHP was shown to regulate glucose metabolism-related genes (IGFBPs and GLUTs families), suggesting that this modulation could lead to cardiomyocyte hypertrophy by triggering aberrant glucose metabolism expression.[43] Furthermore, research suggests that heart failure is caused by changes in energy metabolism, including a significant increase in high-energy-phosphate content, mitochondrial malfunction and a gradual reliance on glucose as a substrate.[47] There was a significant drop in the tissue level of glucose transporters in the experimental groups subjected to 50 g/kg and 500 g/ kg doses of BPA,[48] which agrees with our findings. Similarly, Indumathi et al. discovered that BPA treatment in male adult rats resulted in decreased Akt phosphorylation and lower GLUT4 protein expression in the membrane and cytoplasm.[49] Reduction in cardiac GLUT-4 expression in BPA treated rats reflects systemic hyperglycaemia at the cardiomyocyte level which signifies reduction in glucose uptake in heart tissues of the BPA treated rats.
The enzymes involved in fatty acid intake, mitochondrial fatty acid uptake and fatty acid oxidation are all governed by PPAR, which is abundantly expressed in the heart.[50] Because fatty acids are endogenous PPAR ligands, increased amounts of fatty acid ligands in the bloodstream have the potential to activate PPAR/PGC-1 pathways, resulting in increased fatty acid absorption and oxidation. In this study, the % expression of PPARα increased significantly in BPA (P < 0.05) and BPA + DBP (P < 0.001) when compared to the control. Indeed, in diabetic or insulin-resistant rodent models, cardiac PPAR expression is increased, as demonstrated by a rise in downstream target genes as well as real fatty acid oxidation rates.[51] Fatty acids are a primary energy source for the working heart under physiological conditions, providing around 70% of the ATP needed, with the remaining energy coming from glucose, ketone bodies and branched chain amino acids. However, in diabetic hearts, increased fatty acid uptake and lower glucose utilisation have been seen in both animal models and human patients.[52] In addition, it has been proposed that abnormally elevated cardiac PPAR expression is a key factor in the pathogenesis of diabetic cardiomyopathy. Experimental evidence showing that over-expression of PPARα caused mice to develop a severe cardiomyopathy,[53] whereas inhibition of PPARα prevented the development of diabetic cardiomyopathy, lends support to this idea.[54] Similarly, mice with cardiac-specific PPAR over-expression had similar phenotypes of diabetic cardiomyopathy, left ventricular hypertrophy and systolic dysfunction.[53] In contrast, in PPARα deficient diabetic animals, there were reduced fatty acid oxidation and absorption increases along with an increase in glucose metabolism.[55] Therefore, preventing metabolic changes by modifying cardiac PPARα may be a promising treatment approach for treating diabetic cardiomyopathy.
There was an increase in adenosine monophosphate AMPK expression following administration of the plasticizers as seen in BPA, DBP and BPA + DBP groups (P < 0.01). AMPK is a metabolic stress sensor that controls a variety of physiological and pathological cellular activities.[56] The physiologic actions of mitochondria and the oxidation of fatty acids are stimulated by the phosphorylation and activation of AMPK, which consequently promotes lipid use.[57] Chen et al.[58] discovered that lipid metabolism is influenced by the PPAR/AMPK pathway to some extent. In agreement with our findings, Zhang et al. examined the hepatic levels of total phosphorylated AMPK and found that they were significantly greater in the livers of rats fed a high fat diet and given 300 mg/kg bw DEHP than in the control and high fat diet alone groups.[59] One of the major cellular stresses that AMPK regulate is oxidative stress. BPA and DBP treatment led to elevated cardiac expression of AMPK in the present study, signifying that plasticizers (BPA and DBP) induced cardiac oxidative stress resulting in elevated cardiac AMPK expression. AMPK is triggered when the metabolism of ROS is disrupted, and it increases the expression of antioxidant enzymes to reduce ROS generation.[60] As reported in our research, BPA and DBP induced oxidative stress in the heart tissue,[7] which could partly account for the up-regulation of AMPK in this present study.
Flavonoids have emerged as a promising method for modifying the expression of key metabolic enzymes involved in heart energy metabolism.[61] Interestingly, as observed in this study, Rt reduced blood glucose, increased plasma insulin, and reduced plasma and cardiac FFA (P < 0.05) signifying improved glucometabolic regulation in BPA and DBP treated rats which are in line with previous findings. In streptozotocin-induced diabetic mice, Rt suppressed hyperglycaemia, raised insulin plasma concentrations and reduced oxidative stress.[56,62] Rt, as a polyphenolic flavonoid, may cause intact functional cells to create insulin and/or protect them from further degeneration, allowing them to remain active and release insulin.[63] Rt reduced the fatty components in hypercholesterolemic rats’ serum, most likely through inhibiting HMG-CoA reductase activity.[56,64] Panchal et al. also documented that Rt-treated rats had lower plasma concentrations of total cholesterol, TG, FFA and insulin.[65] This study revealed further that Rt supplementation positively modulates the activities of hexokinase and PDH in the plasticizers treated rats (P < 0.05). Likewise, Rt increased the expression of PPARα and decreased the expression of AMPK (P < 0.05). Hence, cardiac energetic regulation is improved by Rt through modulation of PPARα in myocardial tissues of BPA and DBP treated rats. Impaired cardiac metabolic flexibility associated with cardiac dysfunctions is rescued by Rt. This is consistent with a study that found that activating PPAR-in the early stages of heart failure kept myocardial function and energy levels stable.[66] In a study, Rt-rich Djulis hull crude extract markedly increased the expression of PPARα.[67] Furthermore, Rt consumption reduced adipose tissue size and adipogenic gene expression as well as AMPK activity in epididymal adipose tissue, confirming Rt’s anti-obesity and anti-oxidative properties.[68]
CONCLUSION
This study suggested that BPA shifts the cardiac bioenergetics toward fatty acid utilisation as seen in diabetic cardiomyopathy and DBP shifts the cardiac bioenergetics toward glucose utilisation as seen in cardiac hypertrophy condition. However, Rt treatment improves the impaired cardiac bioenergetics through PPARα and AMPK modulation.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
This work was supported by Bowen University, Iwo, Osun State, Nigeria (Grant reference number; BRE/2020/009).
References
- The adverse cardiac effects of Di(2-ethylhexyl) phthalate and Bisphenol A. Cardiovasc Toxicol. 2014;14:339-57.
- [CrossRef] [PubMed] [Google Scholar]
- Plasticizers and cardiovascular health: Role of adipose tissue dysfunction. Front Pharmacol. 2021;11:626448.
- [CrossRef] [PubMed] [Google Scholar]
- Plastics and health risks. Annu Rev Public Health. 2010;31:179-94.
- [CrossRef] [PubMed] [Google Scholar]
- Multi-strain probiotic ameliorated toxic effects of phthalates and bisphenol A mixture in Wistar rats. Food Chem Toxicol. 2020;143:111540.
- [CrossRef] [PubMed] [Google Scholar]
- Urinary bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation. 2013;125:1482-90.
- [CrossRef] [PubMed] [Google Scholar]
- Possible mechanisms of Di(2-ethylhexyl) phthalate-induced MMP-2 and MMP-9 expression in A7r5 Rat vascular smooth muscle cells. Int J Mol Sci. 2015;16:28800-11.
- [CrossRef] [PubMed] [Google Scholar]
- Rutin prevents cardiac oxidative stress and inflammation induced by bisphenol A and dibutyl phthalate exposure via NRF-2/NF-κB pathway. Life Sci. 2021;284:119878.
- [CrossRef] [PubMed] [Google Scholar]
- Heart metabolism in sepsis-induced cardiomyopathy-unusual metabolic dysfunction of the heart. Int J Environ Res Public Health. 2021;18:7598.
- [CrossRef] [PubMed] [Google Scholar]
- The metabolic disturbances of isoproterenol induced myocardial infarction in rats based on a tissue targeted metabonomics. Mol Biosyst. 2013;9:2823-34.
- [CrossRef] [PubMed] [Google Scholar]
- DNA methylation reprograms cardiac metabolic gene expression in end-stage human heart failure. Am J Physiol Circ Physiol. 2019;317:H674-84.
- [CrossRef] [PubMed] [Google Scholar]
- The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight. 2019;4:e124079.
- [CrossRef] [PubMed] [Google Scholar]
- Phthalate exposure changes the metabolic profile of cardiac muscle cells. Environ Health Perspect. 2012;120:1243-51.
- [CrossRef] [PubMed] [Google Scholar]
- Taxifolin alleviates apoptotic injury induced by DEHP exposure through cytochrome P450 homeostasis in chicken cardiomyocytes. Ecotoxicol Environ Saf. 2019;183:109582.
- [CrossRef] [PubMed] [Google Scholar]
- The influence of polyphenols on metabolic disorders caused by compounds released from plastics-review. Chemosphere. 2020;240:124901.
- [CrossRef] [PubMed] [Google Scholar]
- Rutin alleviates diabetic cardiomyopathy in a rat model of Type 2 diabetes. Exp Ther Med. 2015;9:451-5.
- [CrossRef] [PubMed] [Google Scholar]
- Enzymatic determination of glucose in blood serum. Ann Clin Biochem. 1969;6:24.
- [CrossRef] [Google Scholar]
- Cadmium exposure induces cardiac glucometabolic dysregulation and lipid accumulation independent of pyruvate dehydrogenase activity. Ann Med. 2021;53:1108-17.
- [CrossRef] [PubMed] [Google Scholar]
- Determination of pyruvate dehydrogenase and acetyl-CoA synthetase activities using citrate synthase. Anal Biochem. 1981;115:81-7.
- [CrossRef] [PubMed] [Google Scholar]
- Protective effects and chemical composition of Corchorus olitorius leaf fractions against isoproterenol-induced myocardial injury through p65NFkB-dependent anti-apoptotic pathway in rats. J Basic Clin Physiol Pharmacol. 2020;31 doi: 10.1515/jbcpp-2019-0108
- [CrossRef] [PubMed] [Google Scholar]
- Myocardial substrate metabolism in heart disease. Front Biosci (Schol Ed). 2012;4:556-80.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiac toxicity from bisphenol A exposure in human-induced pluripotent stem cell-derived cardiomyocytes. Toxicol Appl Pharmacol. 2021;428:115696.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of the short term toxicity of phthalate diesters and monoesters in sprague-dawley male rats. Toxicol Res. 2010;26:75-82.
- [CrossRef] [PubMed] [Google Scholar]
- The influence of phthalates and bisphenol A on the obesity development and glucose metabolism disorders. Endocrine. 2017;55:666-81.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term exposure to bisphenol A or S promotes glucose intolerance and changes hepatic mitochondrial metabolism in male Wistar rats. Food Chem Toxicol. 2019;132:110694.
- [CrossRef] [PubMed] [Google Scholar]
- Lycopene: Hepatoprotective and antioxidant effects toward bisphenol A-induced toxicity in female wistar rats. Oxid Med Cell Longev. 2018;2018:5167524.
- [CrossRef] [PubMed] [Google Scholar]
- Oleuropein and hydroxytyrosol rich extracts from olive leaves attenuate liver injury and lipid metabolism disturbance in bisphenol A-treated rats. Food Funct. 2018;9:3220-34.
- [CrossRef] [PubMed] [Google Scholar]
- Boswellic acid protects against Bisphenol-A and gamma radiation induced hepatic steatosis and cardiac remodelling in rats: Role of hepatic PPAR-α/P38 and cardiac calcineurin-A/NFATc1/P38 pathways. Arch Physiol Biochem. 2020;128:767-85.
- [CrossRef] [PubMed] [Google Scholar]
- Bisphenol-A and metabolic diseases: Epigenetic, developmental and transgenerational basis. Environ Epigenet. 2016;2:dvw022.
- [CrossRef] [PubMed] [Google Scholar]
- Bisphenol A exposure may induce hepatic lipid accumulation via reprogramming the DNA methylation patterns of genes involved in lipid metabolism. Sci Rep. 2016;6:31331.
- [CrossRef] [PubMed] [Google Scholar]
- Adverse effects of long-term exposure to bisphenol A during adulthood leading to hyperglycaemia and hypercholesterolemia in mice. Toxicology. 2014;325:133-43.
- [CrossRef] [PubMed] [Google Scholar]
- Distinct cardiac energy metabolism and oxidative stress adaptations between obese and non-obese Type 2 diabetes mellitus. Theranostics. 2020;10:2675-95.
- [CrossRef] [PubMed] [Google Scholar]
- Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol. 2016;12:144-53.
- [CrossRef] [PubMed] [Google Scholar]
- Lipid metabolism and toxicity in the heart. Cell Metab. 2012;15:805-12.
- [CrossRef] [PubMed] [Google Scholar]
- Metformin reverses hexokinase and 6-phosphofructo-1-kinase inhibition in skeletal muscle, liver and adipose tissues from streptozotocin-induced diabetic mouse. Arch Biochem Biophys. 2010;496:53-60.
- [CrossRef] [PubMed] [Google Scholar]
- Pyridine nucleotide regulation of cardiac intermediary metabolism. Circ Res. 2012;111:628-41.
- [CrossRef] [PubMed] [Google Scholar]
- Urinary bisphenol A and obesity in adults: Results from the Canadian Health Measures Survey. Health Promot Chronic Dis Prev Can. 2017;37:403-12.
- [CrossRef] [PubMed] [Google Scholar]
- Association of urinary concentrations of bisphenols with Type 2 diabetes mellitus: A case-control study. Environ Pollut. 2018;243:1719-26.
- [CrossRef] [PubMed] [Google Scholar]
- Exogenous H(2)S switches cardiac energy substrate metabolism by regulating SIRT3 expression in db/db mice. J Mol Med (Berl). 2018;96:281-99.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular mechanisms of cardiac pathology in diabetes-Experimental insights. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1949-59.
- [CrossRef] [PubMed] [Google Scholar]
- The randle cycle revisited: A new head for an old hat. Am J Phys Endocrinol Metab. 2009;297:E578-91.
- [CrossRef] [PubMed] [Google Scholar]
- Metabolic profiling of the diabetic heart: Toward a richer picture. Front Physiol. 2019;10:639.
- [CrossRef] [PubMed] [Google Scholar]
- Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005;146:5341-9.
- [CrossRef] [PubMed] [Google Scholar]
- Taxifolin ameliorates DEHP-induced cardiomyocyte hypertrophy via attenuating mitochondrial dysfunction and glycometabolism disorder in chicken. Environ Pollut. 2019;255:113155.
- [CrossRef] [PubMed] [Google Scholar]
- Double knockout of Akt2 and AMPK accentuates high fat diet-induced cardiac anomalies through a cGAS-STING-mediated mechanism. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165855.
- [CrossRef] [PubMed] [Google Scholar]
- Glucose transporters in cardiac metabolism and hypertrophy. Compr Physiol. 2015;6:331-51.
- [CrossRef] [PubMed] [Google Scholar]
- Glucose metabolism and cardiac hypertrophy. Cardiovasc Res. 2011;90:194-201.
- [CrossRef] [PubMed] [Google Scholar]
- Heart disease and stroke statistics-2016 update: A report from the American heart Association. Circulation. 2016;133:e38-360.
- [Google Scholar]
- Chronic exposure of bisphenol A impairs carbohydrate and lipid metabolism by altering corresponding enzymatic and metabolic pathways. Environ Toxicol Pharmacol. 2020;78:103387.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of bisphenol-A on insulin signal transduction and glucose oxidation in skeletal muscle of adult male albino rat. Hum Exp Toxicol. 2013;32:960-71.
- [CrossRef] [PubMed] [Google Scholar]
- Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207-58.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiac fatty acid oxidation in heart failure associated with obesity and diabetes. Biochim Biophys Acta. 2016;1861:1525-34.
- [CrossRef] [PubMed] [Google Scholar]
- Altered myocardial substrate metabolism and decreased diastolic function in nonischemic human diabetic cardiomyopathy: Studies with cardiac positron emission tomography and magnetic resonance imaging. J Am Coll Cardiol. 2009;54:1524-32.
- [CrossRef] [PubMed] [Google Scholar]
- The cardiac phenotype induced by PPAR alpha over expression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121-30.
- [CrossRef] [PubMed] [Google Scholar]
- Glucagon-like peptide-1 ameliorates cardiac lipotoxicity in diabetic cardiomyopathy via the PPARα pathway. Aging Cell. 2018;7:e12763.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of peroxisome proliferator-activated receptor-α on diabetic cardiomyopathy. Cardiovasc Diabetol. 2021;20:2.
- [CrossRef] [PubMed] [Google Scholar]
- Sonchus oleraceus Linn extract enhanced glucose homeostasis through the AMPK/Akt/GSK-3beta signaling pathway in diabetic liver and HepG2 cell culture. Food Chem Toxicol. 2020;136:111072.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic implication of pAMPK immunohistochemical staining by subcellular location and its association with SMAD protein expression in clear Cell Renal Cell Carcinoma. Cancers (Basel). 2019;11:1602.
- [CrossRef] [PubMed] [Google Scholar]
- Fenofibrate lowers lipid accumulation in myotubes by modulating the PPARα/AMPK/FoxO 1/ATGL pathway. Biochem Pharmacol. 2012;84:522-31.
- [CrossRef] [PubMed] [Google Scholar]
- Disturbance of di-(2-ethylhexyl) phthalate in hepatic lipid metabolism in rats fed with high fat diet. Food Chem Toxicol. 2020;146:111848.
- [CrossRef] [PubMed] [Google Scholar]
- AMP-activated protein kinase: A remarkable contributor to preserve a healthy heart against ROS injury. Free Radic Biol Med. 2021;166:238-54.
- [CrossRef] [PubMed] [Google Scholar]
- Metabolic enzymes dysregulation in heart failure: The prospective therapy. Heart Fail Rev. 2017;22:109-21.
- [CrossRef] [PubMed] [Google Scholar]
- Rutin improves glucose homeostasis in streptozotocin diabetic tissues by altering glycolytic and gluconeogenic enzymes. J Biochem Mol Toxicol. 2006;20:96-102.
- [CrossRef] [PubMed] [Google Scholar]
- Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic wistar rats. Basic Clin Pharmacol Toxicol. 2006;98:97-103.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of rutin on lipid profile in hypercholesterolaemic rats. Basic Clin Pharmacol Toxicol. 2009;104:253-8.
- [CrossRef] [PubMed] [Google Scholar]
- Rutin attenuates metabolic changes, nonalcoholic steatohepatitis, and cardiovascular remodeling in high-carbohydrate, high-fat diet-fed rats. J Nutr. 2011;141:1062-9.
- [CrossRef] [PubMed] [Google Scholar]
- Activation of PPAR-α in the early stage of heart failure maintained myocardial function and energetics in pressure-overload heart failure. Am J Physiol Heart Circ Physiol. 2017;312:H305-13.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-NAFLD effect of Djulis hull and its major compound, rutin, in mice with high-fat diet (HFD)-induced obesity. Antioxidants (Basel). 2021;10:1694.
- [CrossRef] [PubMed] [Google Scholar]
- Rutin increases muscle mitochondrial biogenesis with AMPK activation in high-fat diet-induced obese rats. Nutrients. 2015;7:8152-69.
- [CrossRef] [PubMed] [Google Scholar]







