Translate this page into:
Motor units involvement in diaphragm and intercostal muscles during supine and prone posture, and its relationship with oxygen saturation and perfusion index in healthy young female adults
*Corresponding author: Anupam Bandyopadhyay, Department of Physiology, Serampore College, Serampore, West Bengal, India. baneranupam@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Saba N, Chatterjee P, Bandyopadhyay A. Motor units involvement in diaphragm and intercostal muscles during supine and prone posture, and its relationship with oxygen saturation and perfusion index in healthy young female adults. Indian J Physiol Pharmacol. 2023;67:283-9. doi: 10.25259/IJPP_369_2022
Abstract
Objectives:
Due to gravity and non-gravity variables, human posture influences the distribution of ventilation and perfusion. Gravity enhances the air exchange and posture has an impact on the respiratory muscles’ activity. The goal of the study was to determine the role of neuromuscular activities in respiratory muscles throughout various respiratory states and postures, as well as the degree to which two different lying positions affected the perfusion index (PI) and oxygen saturation (SpO2) level in young, healthy female adults.
Materials and Methods:
Thirty sedentary healthy female college students, age ranges from 18 to 25 years having no history of pulmonary or neuromuscular diseases voluntarily cooperated and participated in this study. Each participant’s diaphragm and intercostal muscles underwent surface electromyography in the supine and prone postures. The heart rate (HR), SpO2, and PI were measured.
Results:
When in the supine position, the diaphragm and intercostal muscles both had greater mean root mean square and maximum voluntary contraction values, which is statistically significant (P < 0.05). However, HR, SpO2 level, and PI between supine and prone postures show negligible changes.
Conclusion:
According to this study, prone posture is advantageous to supine posture since it requires less motor unit activation for proper breathing. Contrarily, the increases in chest cavity capacity are insufficient for the healthy adult female’s calm breathing to result in an increase in SpO2 and PI.
Keywords
Perfusion index
Prone posture
Surface electromyography
Supine posture
Oxygen saturation
INTRODUCTION
Gravity and non-gravity factors affect how pulmonary ventilation is distributed and perfused in humans.[1] Gravity stimulates the air exchange the most.[2] The length of the respiratory muscles is affected by posture, which can impact their ability to breathe.[3] The skeletal system, the elasticity of the soft tissue surrounding the thorax, and the strength of the respiratory system’s working muscles are known to impact these alterations.[4] Numerous research studies have looked into healthy people’s supine respiratory processes. It was also stated in a study that the abdominal organs’ pressure on the diaphragm while reclining causes blood to clog up the pulmonary veins. The lung capacities are comparatively smaller as a result of this upward pushing of the diaphragm, which also enhances venous returns. A reclining position causes the amount of blood in the systemic circulation to shift more toward the pulmonary circulation, which reduces the volume of the thorax and increases the abdomen contents’ upward pressure on the diaphragm, resulting in forced breathing.[5] A healthy person in a lying position was found to have a more significant overall increase in lung capacity than a person in a standing position.[6,7]
Other than the lying position, it has been shown by numerous types of research that various body positions have definite effects on people’s respiratory cycles.[8-11] All of these studies seem to indicate that changes in physical structures and the gravitational force have an impact on lung function. Spirometry was the main methodology used in the investigations. However, very little is known about the neurological characteristics of various respiratory muscles in various positions and how to detect whether any alterations to the chest cavity were taking place. Lung capacity could not be increased without altering the chest cavity and respiratory muscles could not contract without the activation or involvement of motor units.
Following the COVID pandemic, many pneumologists advocated for the benefits of different bed-lying positions and activities that affect various lung capacities and enhance breathing. Various theories suggested that a suitable lying position may have encouraged the opening of more alveoli, increased blood oxygen saturation (SpO2), and improved perfusion index (PI). This study is hypothesised based on the impact of gravity on changes in muscle length and the strength of the working muscles in terms of respiratory system neuromuscular activities. The purpose of this study was to examine the motor unit involvement of the diaphragm and intercostal muscles during supine and prone posture, as well as how it is associated with SpO2 and PI in young, healthy female adults. The specific objectives of this study are to observe and determine the differences in the motor unit firing rate and recruitment in the diaphragm and intercostal muscles during supine and prone posture. Furthermore, it was also aimed to assess the relationship of motor unit involvement with SpO2 and PI.
MATERIALS AND METHODS
Selection of participants
This experiment was carried out at the Department of Physiology, Serampore College. The study comprised adult female college students between the ages of 18 and 25 (21.367 ± 1.586 years). For this study, 30 healthy, sedentary female college students who had never experienced any pulmonary or neuromuscular disease voluntarily participated.
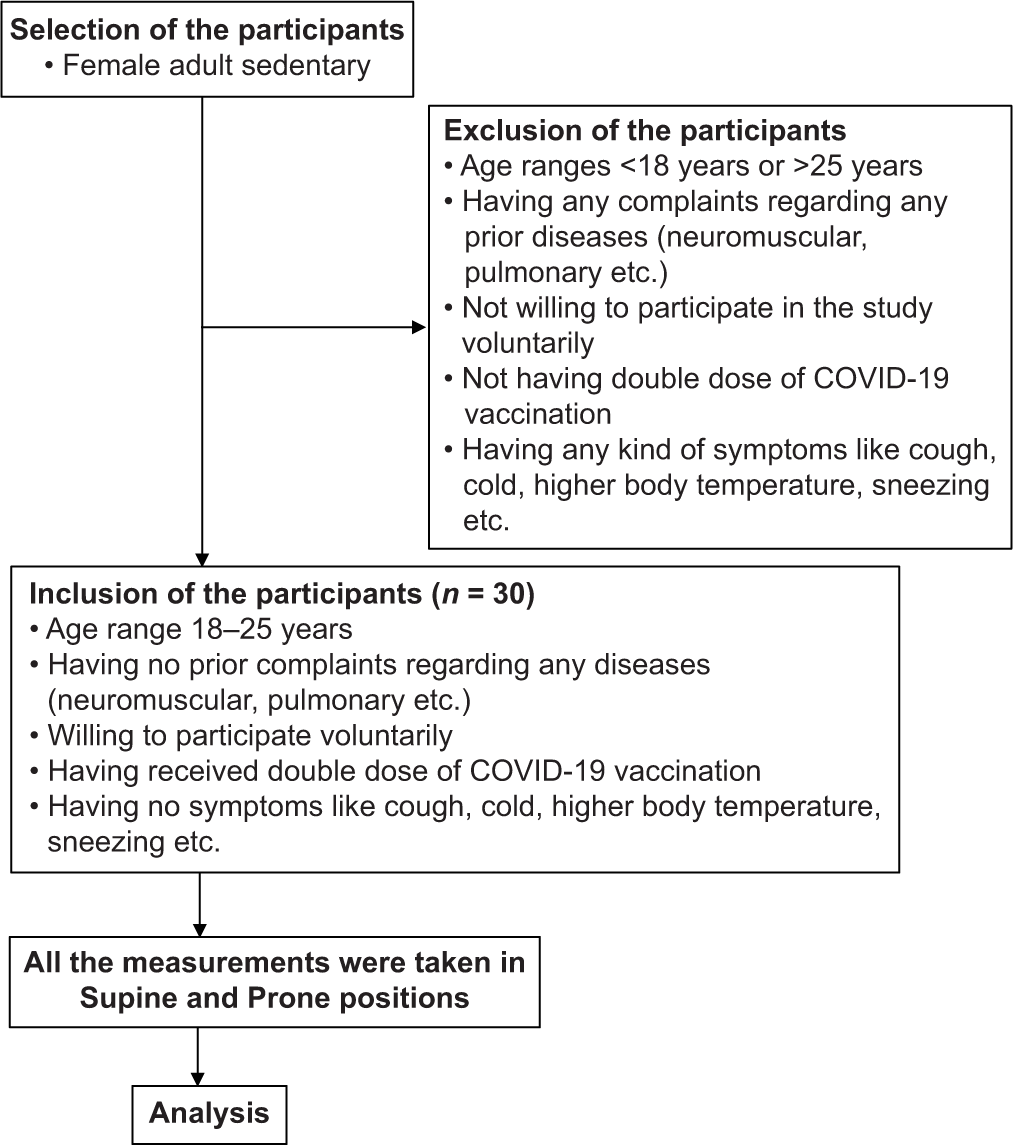
The participants were selected by random sampling method with the proper inclusion and exclusion criteria. This means that each participant had an equal chance of being selected. The recruited participants were from a pool of healthy, sedentary female college students who had never had pulmonary or neuromuscular disease. All of the participants had received two COVID vaccinations and showed no signs of COVID, such as a high body temperature, cough, and sneezing (Refer to the flowchart for the overview with the inclusion and exclusion criteria).
The participants were rested for 10 min in the supine position in the laboratory at 25°C room temperature and a digital hygrometer was used to record the average relative humidity for the 7 days of the experimentation, which ranged from 56% to 68%.
Ethical consideration
The Human Ethical Committee (HEC, Serampore College, affiliated with the University of Calcutta, Serampore, Hooghly, West Bengal, India, with reference number SC/HEC/2022/P1A) approved the study according to the Helsinki Declaration of 1975. The study excluded participants with any respiratory illnesses or muscle limitations as well as those who did not fit the required age range. The participants were given a full explanation of the study’s objectives before the experiment and they provided written consent before participation.
Anthropometric measurements
Stature
Stature was the perpendicular distance between the transverse planes of the Vertex and the inferior aspect of the feet and it was measured by Seca 213 Portable Stadiometer (Seca Deutschland, Hamburg, Germany, range of measurement 60–220 cm). The stature was measured by the ‘Stretch Stature Method’.[12,13]
Weight, lean body mass and fat%
Bioimpedance analysis (BIA) was used to measure weight, lean body mass, and fat percentage. The bioimpedance analyser calculated the body composition. By delivering low-voltage, safe electrical scales to measure the muscle and fat in addition to the total body measurement, Tanita BC601 Innerscan Segmental Body Composition Monitor (Tanita Corporation, Japan) uses the BIA approach to measure body composition with scientifically validated accuracy.[14] The measurements were collected using the standard setting after manually inputting the measured height, weight, gender, and age of the subject.
Body mass index (BMI)
BMI is calculated by dividing body weight in kilogrammes by height in square metres. Since the height measurements were done in centimetres, they were divided by 100 to convert them to metres for calculation. The BMI was measured using the following equation.[15,16]
BMI = kg/m2
Physiological measurements
Respiratory rate
The subject was allowed to lie down while one hand was kept over the chest and the other over the stomach region to measure respiratory rate. They were instructed to breath normally and the number of ups and downs in their hands was tallied to determine their respiratory rate per minute. A full respiratory cycle was thought to be represented by one up and one down.
SpO2, heart rate (HR), and PI
Using a pulse oximeter, blood SpO2, HR, and PI were determined. A pulse oximeter is a tiny, clip-shaped device that may be fastened to the fingers, toes, or earlobes and uses accurate SpO2 measurements to show other data. Before the measurement, the patients were given a 5-min break. The index finger was used to attach the pulse oximeter.[16] The hands were steady and at rest and the fingertip was under the red light. After the reading had steadied for a minute, SpO2, HR, and PI recordings were made.[17]
Electromyographic (EMG) recording
EMG recording kit iWorx 214 (Dover, United States) was set up and prepared for recording. The EMG recording was done on two different respiratory muscles such as the diaphragm and the intercostal muscle. The muscles were isolated and surface button electrodes were placed applying the gel to the skin.[18] On the regions where electrodes have been positioned, the gel was applied. By eliminating air pockets in the stratum corneum of the dermis, the gel enhances the coupling between the electrode and the skin, increasing the signal’s conductivity. The amplifier was linked to the surface EMG electrodes. The participant was instructed to lie down so that the recording could be taken. The following positioning method was used to achieve the collection of surface signals from the diaphragm and intercostal muscle using pairs of surface electrodes:
For the diaphragm, one pair of electrodes was placed in the lowest intercostal spaces on the right side of the body, at the midclavicular line
One pair for the external intercostal muscles, in the fifth intercostal space at the posterior axillary line
A ground electrode was placed on the sternum. The distance between a pair of electrodes was minimum, not more than 2 cm, and care was taken to place the electrodes in the same orientation as the muscle fibres.
EMG recordings were taken in two different postures
Supine posture: The patient is face-up, their head is resting on a pillow and their neck is in a neutral position when they are supine. The patient’s arms may be tucked at the sides or abducted to a lesser degree on arm boards while yet maintaining a neutral thumb-up or supinated position. This is the easiest position because it does not require any specific equipment and is doable.[19]
Prone posture: The medical word for resting flat on your stomach is in a prone posture. Supine position refers to lying flat on one’s back.[20] To conduct the EMG recording, the participants were instructed to lie down in a supine position. Before moving on to the next muscle, the intercostal muscle, the root mean square (RMS) and maximum voluntary contraction (MVC) values of intercostal muscle activity was also taken.
At first, the RMS and MVC values of diaphragm muscle activities were taken. The second stage of the investigation required participants to lie down in the prone position while an EMG recording was made. The RMS and MVC values of the diaphragm muscle activity were initially recorded, and then, the recording was transferred to the intercostal muscle, for which the RMS and MVC values had already been recorded.[21]
Diaphragm EMG and intercostal EMG were done in these two respective conditions. The raw data have been filtered. The RMS and MVC have been measured with the help of the software LabScribe. The EMG Analysis Module for LabScribe software allows various Time domain and frequency domain analyses of the EMG data.[18,22]
Statistical analysis
Statistical analysis of all data was performed by IBM Statistical Package for the Social Sciences version 25. Data are found to be not normally distributed by the Shapiro–Wilk normality assumption. Descriptive statistics were also performed for all the variables to find out the mean and standard deviation. A paired t-test was performed between two conditions (Supine and prone position). P < 0.05 was considered to be a statistically significant result of all assumptions.
RESULTS
Sample characteristics
[Table 1] shows the general characteristics of the 30 healthy young adult female individuals (n = 30) who participated in the study. The average sample age was 21.367 ± 1.586 years. The average fat content of the participants is 32.65 ± 9.48%, which is significantly higher than the recommended range. However, the average BMI of 23.73 ± 3.83 kg/m2 falls into the pre-obese range.
| Variables | Mean±SD |
|---|---|
| Age | 21.367±1.586 |
| Height (cm) | 154.69±6.56 |
| Weight (kg) | 56.99±10.46 |
| BMI (kg/m2) | 23.73±3.83 |
| Lean body mass | 37.49±4.63 |
| Fat % | 32.65±9.48 |
| Respiratory rate (per min) | 17.50±2.162 |
BMI: Body mass index, SD: Standard deviation, n: Number of participants
Physiological variables
The results of the physiological variables are shown in [Table 2]. The HR and PI were found to be statistically insignificant while SpO2 was found to be significant (P < 0.05) between the two postures (Supine and Prone). This means that the HR and PI did not change significantly when the participants changed from a supine to a prone posture, but that the SpO2 did change significantly.
| Variables | Supine | Prone | Paired t-test | |
|---|---|---|---|---|
| Mean±SD | Mean±SD | t-value | Level of significance | |
| HR (bpm) | 86.8±12.84 | 86.63±12.984 | 0.090 | 0.929 (NS) |
| SpO2level | 98.9±0.548 | 98.17±0.648 | 5.809 | 0.000* |
| PI | 4.697±2.57 | 5.17±2.45 | −1.018 | 0.317 (NS) |
NS: Not significant, *P<0.05. HR: Heart rate, SpO2: Oxygen saturation, PI: Perfusion index, SD: Standard deviation, n: Number of participants
EMG variables
[Table 3] displays the outcomes for the myoelectric variables. Between the supine and prone positions, the RMS and MVC of both the diaphragm and intercostal respiratory muscles are found to be statistically significant (P < 0.05). The higher RMS and MVC values for both the respiratory muscles in the supine position than the prone position suggest that in the experimental condition, both the muscles exerted more muscle force or higher motor unit recruitment in the supine condition than in the prone condition.
| Variables | Supine | Prone | Paired t-test | |
|---|---|---|---|---|
| Mean±SD | Mean±SD | t-value | Level of significance | |
| Diaphragm RMS (mV) | 0.131±0.037 | 0.025±0.018 | 24.042 | 0.000* |
| Diaphragm MVC (mV) | 0.206±0.137 | 0.104±0.078 | 6.525 | 0.000* |
| Intercostal RMS (mV) | 0.134±0.022 | 0.038±0.044 | 15.139 | 0.000* |
| Intercostal MVC (mV) | 0.208±0.087 | 0.115±0.121 | 4.494 | 0.000* |
NS: Not significant, *P<0.05. RMS: Root mean square, MVC: Maximum voluntary contraction, SD: Standard deviation, n: Number of participants
DISCUSSION
The study found that the average fat percentage of young females was 32.65 ± 9.48%, which is higher than the World Health Organisation’s recommendation of 25% for adults. The average BMI of the young females was also 23.73 ± 3.83 kg/m2, which is in the pre-obese range.
This means that the majority of the young females in the study are overweight or obese or tend to become obese. The thoracoabdominal kinematics in the supine position are affected by the higher fat content; the abdomen region contributes more to ventilation than the rib cage, suggesting that lung hypoventilation can take place in this position. On the other hand, due to variations in the quantity of lung atelectasis present, pleural pressure rises and ventilation is enhanced in the prone position. The total of all forces compressing the alveolus results in the lung’s pleural pressure. This comprises the pressure exerted by the tissue above the alveolus and the pressure the abdomen exerts on the diaphragm. This is because an alveolus will stay open if the intra-alveolar pressure is higher than the pleural pressure.[23]
The gas exchanges in a typical person are influenced by the prone position. In the prone position, gas exchange is enhanced since ventilation and perfusion heterogeneity are reduced.[24]
According to this study, the usual healthy young female may not experience any changes in HR or PI in either supine or prone positions. The SpO2 level between the supine and prone positions does, however, show a modest change that is significant. However, in both positions, the mean SpO2 level is more than 95%, indicating that all participants had normal blood SpO2 levels. Young, healthy females have the most fat deposition in the hip or subcutaneously, which is unrelated to thoracoabdominal kinematics. The values typically vary from very weak (0.02%) to very strong pulse and the PI is a very excellent indication of pulse strength (20%). One’s general blood supply to the tissue and organs can be inferred from the ratio of pulsing to non-pulsing blood.[25]
The measurements of SpO2, HR, and PI together are effective haemodynamic variables that are non-invasively reflected through it in general and in a pandemic situation like COVID-19.
PI and HR between the supine and prone positions were not found to differ in this investigation. It might be because the thoracic cavities did not alter enough from the supine to the prone posture. The >95% value indicated that there was no issue with the SpO2 level, even though there was a tiny but significant difference in the saturation levels. One possibility is that the weight of the abdominal contents compresses the lungs in the prone position as the participants have significantly higher fat content in their bodies, which can reduce the amount of oxygen that can be taken in. Another possibility is that the diaphragm is less active in the prone position, which can also reduce the amount of oxygen that can be taken in. Although, the difference in SpO2 between the two postures was very mild suggesting that these effects are small. However, even small changes in SpO2 can be important, especially for people with respiratory problems such as chronic obstructive pulmonary disease and asthma.
The fact that the difference in SpO2 between the two postures was very mild suggesting that these effects are small. However, even small changes in SpO2 can be important, especially for people with respiratory problems.
The diaphragm is the main muscle of inspiration and it is responsible for pulling the diaphragm down and increasing the volume of the thoracic cavity. In the supine position, the diaphragm has to work harder to overcome the weight of the abdominal contents, which is why it has a higher EMG activity than in prone conditions. The intercostal respiratory muscles are responsible for moving the ribs up and down, which also helps to increase the volume of the thoracic cavity. In the supine position, the intercostal respiratory muscles have to work harder to overcome the weight of the rib cage, which is why they have a higher EMG activity than in prone conditions.
These two important respiratory muscles, that is, the diaphragm, and intercostals are innervated by the phrenic and intercostal nerves, respectively. While the diaphragm splits the chest cavity from the abdominal cavity, these two muscles mostly occupy the thoracic cavity. The diaphragm is pressed against the thoracic and abdominal organs, which also obstruct breathing throughout a human being’s typical respiratory cycle.
According to this study, the diaphragm and intercostal muscle generate more force in the supine position than in the prone position, showing that more motor units are activated for typical inspiration and expiration in the supine condition. It might be because the enhanced abdomen and decreased rib cage contribution helped to overcome the lung hypoventilation scenario. However, if someone lies in a prone posture, fewer motor units participate in the same breathing pattern, which means that the muscles do not recruit as many motor units as the supine condition, so the muscles become relaxed. Therefore, normal breathing in prone posture is more advantageous and effective than in supine posture in terms of the neuromuscular activity of the two primary muscles, but not enough to provide that much room for improving SpO2 and PI. This study makes a compelling case that normal breathing alone while lying flat is insufficient to improve SpO2. Researchers already found that a prone position was beneficial for people who suffer from acute respiratory distress compared to a supine position due to factors such as less lung compression, better gas exchange, and redistribution of blood and airflow.[26] Our study also establishes the same by justifying them from the respiratory muscle point of view. Earlier finding was also that prone positioning does not adversely impact the respiratory mechanics, but may improve them.[27] Hence, the relaxed or less muscle force in a prone position also justifies this one to recommend that a prone position might be useful as it does not create stress in the respiratory muscles as per our study.
CONCLUSION
There are a lot of recommendations and guidelines for improving lung ventilation to get more active alveoli for better gaseous exchange during the COVID-19 pandemic. Experts recommend several sleeping strategies and breathing exercises to accomplish this objective. The results showed that the diaphragm and intercostal muscles had higher MVC and RMS in the supine condition than in the prone condition. This suggests that the diaphragm and intercostal muscles have to work harder to overcome the weight of the abdominal contents and rib cage in the supine position. The results also showed that there was the very mild difference in SpO2 between the two postures with a trend toward a slightly higher SpO2 in the supine condition. This suggests that the supine position may be slightly more beneficial for oxygen uptake than the prone position. The results of this study have implications for the understanding of the mechanisms of respiratory muscle activation and the relationship between posture and respiratory function. The findings may also be relevant to the development of interventions to improve respiratory function in people with respiratory problems. The majority of this type of research used spirometry to evaluate various lung volumes. This is the only study that incorporated the neuromuscular involvement of the two primary muscles in two different lying positions to determine the physiological causes of thoracic cavity volume variations.
According to this study, prone posture is preferable to supine posture since it requires less motor unit activation for proper breathing and does not stress muscles. Contrarily, the increases in chest cavity capacity are insufficient for the healthy adult female’s calm breathing to result in an increase in SpO2 and PI. However, the sample size is limited to interpret these findings for a larger population. Hence, further investigations are also needed.
Acknowledgments
The authors would like to acknowledge the institution. Institutional Ethical Committee and the participants who voluntarily took part in this study for their cooperation.
Ethical approval
The Human Ethical Committee (HEC, Serampore College, affiliated with the University of Calcutta, Serampore, Hooghly, West Bengal, India, with reference number SC/HEC/2022/P1A) approved the study according to the Helsinki Declaration of 1975.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Long-term spiroergometric studies of paraplegics during the clinical period of rehabilitation. Paraplegia. 1973;11:105-10.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiovascular/pulmonary essentials: Applying the preferred physical therapist practice patterns. Phys Ther. 2008;88:1233-4.
- [CrossRef] [Google Scholar]
- Role of the medial medullary reticular formation in relaying vestibular signals to the diaphragm and abdominal muscles. Brain Res. 2001;902:82-91.
- [CrossRef] [PubMed] [Google Scholar]
- Fall in vital capacity with posture. Br J Dis Chest. 1985;79:267-71.
- [CrossRef] [PubMed] [Google Scholar]
- The changes of respiratory functions following postures in cerebral palsy: Spastic diplegia. J Korean Phys Ther. 2004;16:115-28.
- [Google Scholar]
- Effect of posture on vital capacity. J Appl Physiol (1985). 1986;61:1882-4.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of different sitting postures on lung capacity, expiratory flow, and lumbar lordosis. Arch Phys Med Rehabil. 2006;87:504-9.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of side lying on lung function in older individuals. Phys Ther. 1999;79:456-66.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of different positions on FVC and FEV1 measurements of asthmatic patients. J Phys Ther Sci. 2014;26:591-3.
- [CrossRef] [PubMed] [Google Scholar]
- International standards for anthropometric assessment. Vol 137. Potchefstroom, South Africa: ISAK; 2011.
- [Google Scholar]
- Electromyographic representation of vastus lateralis in volleyball players and its relationship with lower limb anthropometric measurements. Int J Kinanthropometry. 2022;2:31-9.
- [CrossRef] [Google Scholar]
- The tanita SC-240 to assess body composition in pre-school children: An evaluation against the three component model. Nutrients. 2016;8:371.
- [CrossRef] [PubMed] [Google Scholar]
- Dental caries experience and use of dental services among preschool children in Ajman, UAE. Int J Paediatr Dent. 2006;16:257-62.
- [CrossRef] [PubMed] [Google Scholar]
- Home isolation & care for COVID-19, How to use a pulse oximeter at home New Delhi: Indian Council of Medical Research; 2020.
- [Google Scholar]
- Pulse oximetry at high altitude. High Alt Med Biol. 2011;12:109-19.
- [CrossRef] [PubMed] [Google Scholar]
- Formulation of regression equation on the basis of endurance time and fatigue indices in biceps brachii by using surface-EMG of 14-19 years aged trained male volleyball players. Int J Phys Educ Sport Health. 2021;8:6-10.
- [CrossRef] [Google Scholar]
- Risks and benefits of patient positioning during neurosurgical care. Anesthesiol Clin. 2007;25:631-53. x
- [CrossRef] [PubMed] [Google Scholar]
- Effect of prone positioning during mechanical ventilation on mortality among patients with acute respiratory distress syndrome: A systematic review and meta-analysis. CMAJ. 2014;186:E381-90.
- [CrossRef] [PubMed] [Google Scholar]
- Diaphragm and intercostal surface EMG and muscle performance after acute inspiratory muscle loading. Respir Physiol Neurobiol. 2007;155:213-9.
- [CrossRef] [PubMed] [Google Scholar]
- An explanatory study of the parameters to be measured from. Int J Eng Comput Sci. 2013;2:207-13.
- [Google Scholar]
- Does prone positioning improve oxygenation and reduce mortality in patients with acute respiratory distress syndrome? Can Respir J. 2014;21:213-5.
- [CrossRef] [PubMed] [Google Scholar]
- Gas exchange in the prone posture. Respir Care. 2017;62:1097-110.
- [CrossRef] [PubMed] [Google Scholar]
- Observations on a new non-invasive monitor of skin blood flow. Clin Exp Pharmacol Physiol. 1989;16:403-15.
- [CrossRef] [PubMed] [Google Scholar]
- Prone positioning for acute respiratory distress syndrome (ARDS) JAMA. 2020;324:1361.
- [CrossRef] [PubMed] [Google Scholar]
- Prone position in acute respiratory distress syndrome. Eur Respir J. 2002;20:1017-28.
- [CrossRef] [PubMed] [Google Scholar]







