Translate this page into:
Nerve conduction velocities in radiologic technologists: A pilot study
*Corresponding author: Virandra Verma, Associate Professor, Department of Physiology, Gara Raja Medical College, Balwant Nagar, Gwalior - 474 002, Madhya Pradesh, India. virendra.verma.vv78@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Singhal S, Jain G, Arya P, Verma V, Rajput AS. Nerve conduction velocities in radiologic technologists: A pilot study. Indian J Physiol Pharmacol 2020;64(4):293-7.
Abstract
Objectives:
Radiologic technologists (RTs) are typically exposed to low doses of radiations for longer periods, which have a health risk over many organs and tissues. Resistant tissues like nerves have shown neuropathic changes due to acute high-dose radiation exposure in the form of radiation therapy but the effect of low-dose chronic radiation exposure over peripheral nerves in RTs has been studied scantily.
Materials and Methods:
Nerve conduction parameters were recorded from 30 RTs and 30 age- and sex-matched healthy individuals who were not exposed to radiation. Motor nerve conduction study (NCS) of bilateral median, ulnar, radial, common peroneal and tibial nerves and sensory NCS of bilateral median, ulnar and radial nerves were recorded and compared.
Results:
Significant changes were observed in the form of reduction in motor and sensory nerve conduction velocity (P < 0.05) in all the examined nerves. Sensory nerve action potential (SNAP) amplitudes were reduced and latencies were prolonged significantly (P < 0.05) in all the examined sensory nerves. We also found reduced compound muscle action potential amplitude (significant in ulnar, radial, common peroneal and tibial nerves) along with prolonged motor distal latencies (significant in median, ulnar and tibial nerves) among RTs compared to healthy individuals.
Conclusion:
Chronic low-dose exposure of ionising radiation causes sub-clinical neuropathies affecting both sensory and motor nerves.
Keywords
Compound muscle action potential amplitude
Latency
Nerve conduction study
Occupational ionising radiation
Sensory nerve action potential amplitude
INTRODUCTION
Nerve conduction study (NCS) is most commonly used in neurophysiological laboratories not only for the understanding of normal peripheral nerve structure and function but also in relation to various diseases. NCS is an objective test, which involves electrical stimulation of a nerve and recording of the evoked potential either from the nerve itself or from the muscle.[1] Abnormal nerve conduction may be caused by various pathological processes, which hamper fast conduction such as damage or loss of myelin, focal compression (carpal tunnel syndrome), axonal loss or generalised peripheral neuropathy.[2] NCS includes assessment of motor and sensory action potentials, namely, compound muscle action potential (CMAP) in motor nerves and sensory nerve action potentials (SNAP) in sensory nerves. Commonly measured parameters of CMAP and SNAP include distal latency, amplitude, conduction velocity and duration.[3] Different pathological processes result in changes in NCS parameters.
Tissue changes due to radiation exposure result in inflammation and fibrosis that affect the peripheral nerve and lead to peripheral neuropathy.[4] Similar changes have been studied by many authors previously in patients exposed to radiotherapy.[5-7]
Since the introduction of numerous new radiologic procedures, uses of radiation have increased in modern medicine. Radiation exposure of radiologic technologists (RTs) is about 2 times higher than that of other occupation groups in the fields of diagnostic radiation workers, such as physicians, dentists, dental hygienists and nurses. During therapeutic and diagnostic medical procedures, the extremities of RTs are especially highly exposed to X-rays. RTs are typically exposed to low doses of radiations for longer periods, which have a health risk over many organs and tissues.[8]
Effects on NCS due to radiation therapy are well known. However, alterations in NCS parameters in RTs have not yet been reported properly. Hence, we tried to study the effect of chronic radiation exposure on peripheral NCS parameters of upper and lower limb nerves in radiology staff working in the Jaya Arogya Group of Hospitals, Gwalior.
MATERIALS AND METHODS
The current study was conducted in the Department of Physiology, G R Medical College and JA Group of Hospitals, Gwalior. Thirty (26 males and 4 females) RTs with age between 30 and 60 years old who were occupationally exposed to long-term low doses of ionising radiation and having a history of at least 3 years exposure in radiology were recruited. The selected cases were compared with another group of 30 (24 males and 6 females) healthy participants from same institute, who were not exposed to radiations for 1 year as a control group.
The exposed group was matched with controls in age, sex and body mass index. Cases included from different types of imaging modalities and equipment, including conventional and computed tomography and computed radiography. Cases worked in different shifts for 8 h a day for 6 days/week.
The Institutional Ethical Committee approval (No.- 543/ bio/mc/ethical, dated 714/18) was taken. Before enrolment, informed and written consent was taken from all the patients and explained the procedure in local language.
Exclusion criteria
Participants who had any previous diseases such as gross anaemia, known history of diabetes mellitus, cardiopulmonary disease, acute or chronic infection, autoimmune disease and malignancy were excluded from the study to rule out the possible other aetiology for neural affection. Furthermore, participants with <3 years of exposure were excluded from the study.
NCS – in all subjects, NCS was performed by computerised RMS EMG EP Mark-II machine, Panchkula, Haryana (India). Motor and sensory nerve conduction studies were done in all subjects which included the determination of motor and sensory nerve conduction velocity (NCV), amplitude and distal motor latencies of median, ulnar and radial nerve in bilateral upper limbs along with motor NCV, amplitude and distal motor latencies of common peroneal and tibial nerves in bilateral lower limbs. For motor conduction studies, gain was set at 5 mv per division for median, ulnar and tibial nerves and at 2 mv per division for radial and peroneal nerves. The duration of the electrical pulse was set at 100 μs and nerves were stimulated using a current in the range from 20 to 50 mA to achieve supramaximal stimulation.
While for sensory conduction studies, the gain was set at 10 μV per division, electrical pulse of 100 μs duration was used and nerves were stimulated using a current in the range from 15 to 30 mA to achieve supramaximal stimulation.
Ground electrodes were placed between stimulating and recording electrodes. For motor conduction studies, surface active electrodes were placed over the muscle belly and reference over the tendon of abductor pollicis brevis for median nerve, abductor digiti minimi for ulnar nerve, extensor digitorum indicis for radial nerve and extensor digitorum brevis for common peroneal and abductor hallucis for tibial nerve. For sensory studies, ground is placed over dorsum of the hand and the active ring electrode was placed over the 1st digit for radial nerve, 2nd digit for median nerve and 5th digit for ulnar nerve and reference nearly 2–3 cm distally in sensory nerves.
Single supramaximal stimulus given for motor recording and 20 supramaximal stimuli were averaged for smooth recording of sensory nerve and to remove artefacts.
For motor studies, distance between the proximal and distal stimulating sites in mm and in sensory studies distance between the active electrode and stimulating electrode in mm was used to calculate the conduction velocities. RMS machine calculated the velocities automatically on feeding the distance in mm.
Statistical analysis
Statistical analysis was done by descriptive and inferential statistics using Student’s unpaired t-test to compare between cases and controls. P< 0.05 was considered as statistically significant. Statistical analysis was done using GraphPad Prism version 5.01 software by unpaired ‘t-test’ for various analyses.
RESULTS
Nerve conduction parameters of 30 RTs (30–60 years; mean age 43.27 ± 10.1 years) with mean duration of occupational radiation exposure 18.73 ± 12.19 years were compared with 30 control subjects (30–60 years; mean age 42.17 ± 8.46 years) and were analysed. There was no significant difference between the right and left side of nerve parameters of each nerve both in cases and controls separately hence for analysis each subject contributed two data pertaining to his right and left sides for every nerve.
Motor nerves
Motor nerve distal latencies
The difference of distal latency of motor nerves among cases and control subjects in median, ulnar and tibial nerves was statistically significant, where cases had significantly higher distal latencies compared to control subjects.
However, the difference of distal latency of radial nerve and common peroneal nerve was not statistically significant, but cases had higher distal latencies compared to control subjects [Table 1].
| Motor distal latencies | |||
|---|---|---|---|
| Distal latency (ms) | Cases (n=60) mean±SD | Control (n=60) mean±SD | P-value |
| Median | 3.02±0.47 | 2.82±0.39 | 0.016* |
| Ulnar | 2.50±0.58 | 2.17±0.56 | 0.002* |
| Radial | 1.81±0.78 | 1.59±0.40 | 0.052 |
| Common peroneal | 3.73±0.77 | 3.45±0.72 | 0.067 |
| Tibial | 5.25±1.39 | 4.67±1.03 | 0.011* |
| Sensory latencies | |||
| Latency (ms) | Cases (n=60) mean±SD | Control (n=60) mean ±SD | P-value |
| Median | 2.71±0.43 | 2.44±0.28 | 0.000* |
| Ulnar | 2.29±0.33 | 2.14±0.30 | 0.014* |
| Radial | 1.87±0.34 | 1.67±0.30 | 0.001* |
CMAP amplitudes
Cases had lower CMAP amplitude compared to control subjects in all the examined motor nerves. Statistically significant difference was noted in ulnar, radial, common peroneal and tibial nerves [Table 2].
| CMAP amplitude | |||
|---|---|---|---|
| CMAP amplitude (mV) | Cases (n=60) mean±SD | Control (n=60) mean±SD | P-value |
| Median | 11.51±3.78 | 12.22±3.87 | 0.316 |
| Ulnar | 8.57±2.22 | 11.14±3.62 | 0.000* |
| Radial | 3.83±1.93 | 4.89±2.23 | 0.006* |
| Common peroneal | 6.35±2.30 | 7.41±2.45 | 0.015* |
| Tibial | 6.48±4.39 | 9.43±4.20 | 0.000* |
| SNAP amplitude | |||
| SNAP amplitude (µV) | Cases (n=60) mean±SD | Control (n=60) mean±SD | P-value |
| Median | 30.47±14.56 | 43.22±20.83 | 0.000* |
| Ulnar | 20.24±12.20 | 29.59±17.16 | 0.000* |
| Radial | 23.91±9.19 | 29.35±15.85 | 0.023* |
Motor NCV
The difference of NCV of motor nerves among cases and control subjects in all the examined motor nerves, namely, median (cases – 56.92 ± 6.08 m/s and controls – 59.99 ± 3.94 m/s; P = 0.001), ulnar (cases – 57.10 ± 6.94 m/s and controls – 60.67 ± 9.43 m/s; P = 0.02), radial (cases – 53.29 ± 8.81 m/s and controls – 59.55 ± 7.24 m/s; P < 0.001), common peroneal (cases – 47.90 ± 5.16 m/s and controls – 51.55 ± 5.52 m/s; P < 0.001) and tibial nerves (cases – 44.23 ± 5.16 m/s and controls – 47.12 ± 5.79 m/s; P = 0.004) was statistically significant, where cases had significantly reduced conduction velocity compared to control subjects [Figure 1].

- Conduction velocity of motors in cases and controls. #CP N = Common peroneal nerve, *Significant P-value.
Sensory nerves
Sensory nerve latencies
The difference of latency of sensory nerves among cases and control subjects in median, ulnar and radial nerves was statistically significant, where cases had significantly higher latencies compared to control subjects [Table 1].
SNAP amplitudes
Cases had significantly reduced SNAP amplitude compared to control subjects in all examined sensory nerves, namely, median, ulnar and radial nerves [Table 2].
Sensory NCV
The difference of NCV of sensory nerves among cases and control subjects in all the examined sensory nerves, namely, median (cases – 52.38 ± 7.76 m/s and controls – 56.79 ± 5.82 m/s; P < 0.001), ulnar (cases – 51.74 ± 6.16 m/s and controls – 58.53 ± 6.44 m/s; P < 0.001) and radial nerves (cases – 55.63 ± 9.45 m/s and controls – 63.61 ± 9.74 m/s; P< 0.001) was statistically significant, where cases had significantly reduced conduction velocity compared to control subjects [Figure 2].
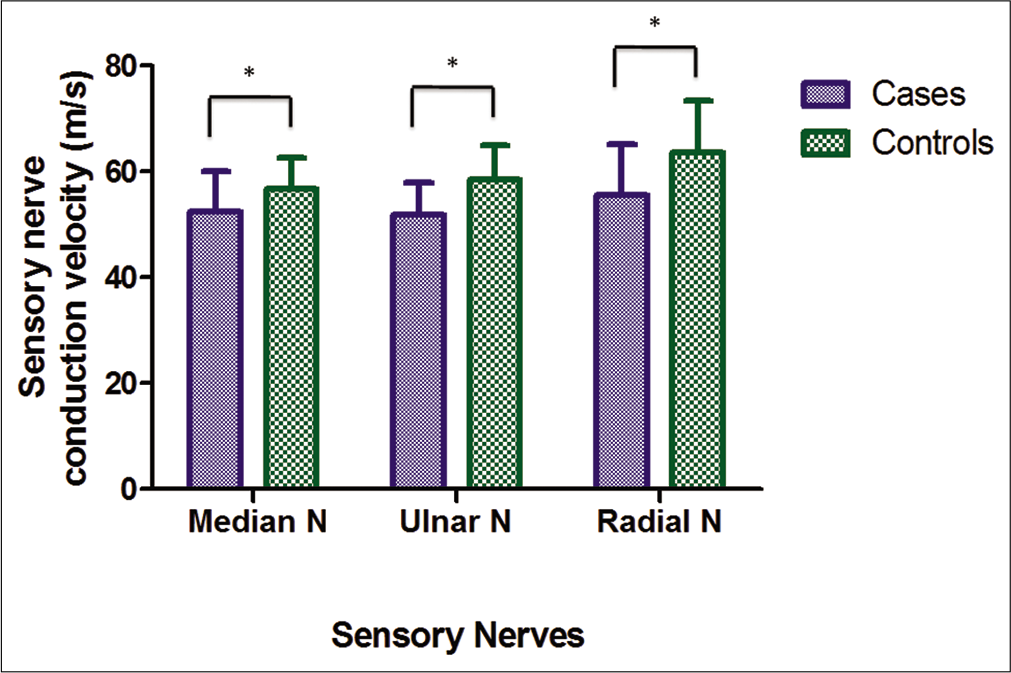
- Conduction velocity of sensory nerves in cases and controls. *Significant P-value.
This figure shows the comparison of conduction velocities in motor nerves among cases and controls. There was significantly (P < 0.05) reduced conduction velocity among cases in all the above nerves tested.
This figure shows the comparison of conduction velocities in sensory nerves in cases and controls. There is significant (P < 0.05) decrease shown in all nerves.
DISCUSSION
The present study was conducted to assess the effects of chronic low-dose ionising radiation exposure over NCS parameters. We observed significant (P< 0.05) changes in the form of reduction in motor and sensory nerve conduction velocities in all the examined nerves. We also observed that SNAP amplitudes were reduced and latencies were prolonged significantly (P < 0.05) in all the examined sensory nerves. Further, we found reduced CMAP amplitudes (significant in ulnar, radial, common peroneal and tibial nerves) along with prolonged motor distal latencies (significant in median, ulnar and tibial nerves) among RTs compared to healthy individuals. Thus, all the parameters were significant in each of the examined sensory nerve and were significant in some of the motor nerves. However, there was a trend of prolonged latencies along with trend of reduced amplitudes and conduction velocities in RTs compared to healthy individuals in all the examined motor and sensory nerves (figures and tables).
Neuropathic changes have been observed in patients undergoing radiotherapy by high-dose acute radiation, which is similar to our study explaining deteriorating changes in peripheral nerves of RTs.
Pathologically, delayed radiation-induced peripheral nerve damage is characterised by fibrosis, vascular lesions and parenchymal damage leading to both axonal and demyelinating changes.[9] These changes affect nerve conduction parameters in the form of prolonged latency and reduced amplitude and conduction velocity.
Microscopically, there are extensive loss of myelin sheath, nerve atrophy and fibrous replacement of nerve fibrils which were seen in patients undergone radiotherapy. Post-irradiation neuropathy involving the brachial and cervical plexuses developed sensory and motor symptoms in upper limbs.[5,10]
Radiation effects are persistent and have very less chances of recovery. This may be the reason of the findings of our study, where even low-dose radiations could produce significant effects on NCS parameters in long term. Painful sensory symptoms, sensory loss and motor weakness can be found years after radiation treatment.[11] Reduced amplitude of SNAP of the radial, median and ulnar nerves was found even 26 years after the radiation therapy for Hodgkin’s lymphoma.[7]
Lower limb involvement is less common with radiation but neurological deficit in lower limb may indicate vertebral compression with underlying radiation-induced vertebral osteoporosis. Pathologically Schwann cells can be injured by lower radiation dose than neurons. Radiations cause damage to nerve fibres which affect the endoneurium, neurolemma and the axon that lead to motor deficits which include paresis of a group of muscles and complete paralysis of the arm develop in acute radiation exposure also involvement of lower limb after low-dose radiotherapy.[12]
In our study, predominant effect was observed on conduction velocity which was significantly lowered in all the examined motor and sensory nerves of RTs. De Carolis et al. (1986) in their case study also showed that 7 months after the radiotherapy for pheochromocytoma, case suffered painful cramps in the legs and progressive bilateral leg weakness. Motor distal latencies along the common peroneal and tibial nerves were prolonged by 51% and 40% and motor conduction velocity nerves were reduced by 24% and 14%, respectively, after radiotherapy. This is suggestive of demyelinating lesion in nerves.[6]
We also observed affection of motor nerve parameters of lower limb in our study like in a study done by Feistner et al. (1989), which showed that in radiation-induced radiculoplexopathy, there is insidious onset of neurological signs of the motor nerve damage predominantly.[13]
The previous studies have been focused on localised peripheral neuropathy with acute high-dose radiation exposure in the form of radiotherapy. These studies included focal exposure on a particular part of body and assessed the nerves localised to the area of exposure. However, in the current study, we focused primarily over chronic low-dose radiation exposure which also depicted the similar kind of changes but of lower intensity as they did not produce clinical symptoms.
In this study, we could not relate the results with radiation dosage because of inadequate radiation dose monitoring.
CONCLUSION
We conclude that ionising radiations are harmful to all the body tissues including the peripheral nerves, where continuous low-dose exposure leads to significant damage to both motor and sensory nerves. However, lead apron was routinely used by subjects but it cannot cover the examined peripheral nerves. There is a need for further equipment in consideration of peripheral nerves protection, appropriate education and training of personnel in principles of radiological health risk and practice of minimum exposure is highly recommended.
As very little research work has been done over radiation exposure on peripheral nerves which is further less on occupational radiation exposure, we recommend further detailed long-term cohort studies to get deeper insight into the possible pathophysiological mechanisms associated behind these neuropathic changes.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial Support and Sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Comparison of NCV of right and left limb in right handed male subjects. Ann Int Med Dent Res. 2016;2:228-31.
- [CrossRef] [Google Scholar]
- Effect of Table Tennis as recreational sport on upper limb nerve conduction velocity. J Contemp Med Dent. 2015;3:29-32.
- [CrossRef] [Google Scholar]
- Radiation-induced peripheral neuropathies: Etiopathogenesis, risk factors, differential diagnostics, symptoms and treatment. Arch Oncol. 2007;15:81-4.
- [CrossRef] [Google Scholar]
- Radiation-induced peripheral neuropathy. Br Med J. 1966;1:834-7.
- [CrossRef] [PubMed] [Google Scholar]
- Isolated lower motoneuron involvement following radiotherapy. J Neurol Neurosurg Psychiatry. 1986;49:718-9.
- [CrossRef] [PubMed] [Google Scholar]
- Radiation-induced conduction block: Resolution following anticoagulant therapy. Muscle Nerve. 2005;31:642-5.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer risk in diagnostic radiation workers in Korea from 1996-2002. Int J Environ Res Public Health. 2013;10:314-27.
- [CrossRef] [PubMed] [Google Scholar]
- Chapter 5: Pathogenetic mechanisms of selected late effects: Initiation of non-neoplastic late effects: The role of endothelium and connective tissue. Stem Cells. 1995;13:248-56.
- [CrossRef] [PubMed] [Google Scholar]
- Cervical plexus lesions following post-operative radiation therapy of mammary carcinoma. Acta Radiol Ther Phys Biol. 1972;11:209-16.
- [CrossRef] [PubMed] [Google Scholar]
- Radiation-induced brachial plexus injury: Follow-up of two different fractionation schedules. Radiother Oncol. 1990;18:213-20.
- [CrossRef] [Google Scholar]
- Late radiation injury to peripheral nerves. Handb Clin Neurol. 2013;115:743-58.
- [CrossRef] [PubMed] [Google Scholar]
- Post-irradiation lesions of the caudal roots. Acta Neurol Scand. 1989;80:277-81.
- [CrossRef] [PubMed] [Google Scholar]






