Translate this page into:
Oesophageal pressure topographic metrics in refractory gastroesophageal reflux disease: An Indian perspective
*Corresponding author: Anirban Trigunes Bhattacharya, Department of Physiology, Armed Forces Medical College, Pune, Maharashtra, India. drbhatta005@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bhattacharya AT, Dua S, Chawla A, Dey D. Oesophageal pressure topographic metrics in refractory gastroesophageal reflux disease: An Indian perspective. Indian J Physiol Pharmacol. 2024;68:231-6. doi: 10.25259/IJPP_31_2024
Abstract
Objectives:
Gastroesophageal reflux disease (GERD) is usually caused by dysfunction of the lower oesophageal sphincter (LES). However, abnormal patterns of oesophageal motility, such as ineffective oesophageal motility (IEM) or absent peristalsis, leading to impaired oesophageal clearance may also cause or aggravate GERD, leading to refractoriness to treatment. The objective of this study was to analyse oesophageal topographic metrics in patients presenting with symptoms of GERD, refractory to treatment.
Materials and Methods:
A retrospective analysis of 30 patients who presented with refractory heartburn/regurgitation of 06 months–03 years duration was done. pH metry (DeMeester score) was analysed. Sixteen channel high-resolution manometry (HRM) was used to study the oesophageal motility. Basal LES pressure (BLESP), integrated relaxation pressure (IRP) and 5 mL water swallows were assessed to determine the type of oesophageal peristalsis based on Chicago Classification version 4.0. The Statistical Package for the Social Sciences version 20 was used to analyse the data. Descriptive statistics such as percentage, mean, and standard deviation were reported. Karl-Pearson’s correlation was used to determine the correlation between age, BLESP and IRP.
Results:
On analysis of 30 patients, 14 (46.66%) patients were found to have normal LES pressure and normal peristaltic wave, 07 (23.33%) patients showed hypotensive LES with normal peristaltic wave, and 09 (30%) were found to have IEM.
Conclusion:
About one-third of patients in our study showed IEM. HRM must be considered in patients with GERD, especially those who are refractory to treatment.
Keywords
Oesophageal peristalsis
Gastroesophageal reflux disease
High-resolution manometry
INTRODUCTION
Gastroesophageal reflux disease (GERD) is characterised by the reflux of gastric contents into the oesophagus, leading to symptoms of heartburn and regurgitation, which may be associated with mucosal damage and dysmotility.[1] While the prevalence of reflux disease is far more than the peristaltic defects, there seems to be a cause-effect relationship between GERD and oesophageal motility disorders. Disruption of the mucosal barrier can lead to defects in oesophageal peristalsis which may eventually progress to motility disorders.[2] On the other hand, impaired oesophageal motility can cause the inability of the oesophagus to clear refluxate and the development of gastroesophageal reflux. However, the direction and strength of relationship between GERD and oesophageal motility patterns is not well understood.[3]
Several structural and functional mechanisms protect against the occurrence of GERD. Structural anti-reflux barrier, consisting of the lower oesophageal sphincter (LES) at the oesophagogastric junction (EGJ), prevents reflux of the gastric contents into the oesophagus, while oesophageal peristalsis helps to remove the refluxate and decrease the exposure of the oesophageal mucosa to gastric acid. As a result, disruption of either EGJ and/or oesophageal clearance may lead to GERD.[3] Structural abnormalities of EGJ can also occur due to LES pressure abnormalities like hypotensive LES, while a functional impairment can occur due to frequent transient LES relaxations.[4,5] Further, oesophageal motility abnormalities, such as ineffective or failed peristalsis, can lead to impaired oesophageal clearance, which may subsequently present as GERD.[6] As high-resolution manometry (HRM) finds wider use across clinical settings, physicians may use this modality to study oesophageal motility patterns in patients of GERD.[7]
Recent studies report that oesophageal motility diseases may be prevalent in reflux disorders across a spectrum of non-erosive reflux disease (NERD) to erosive reflux disease (ERD).[8] However, with the advent of HRM and Chicago Classification guidelines, the abnormalities of the peristaltic wave have been deciphered more objectively based on contraction vigour and pattern. The Chicago Classification version 4.0 (CCv4.0) describes various oesophageal topographic metrics in a clinically relevant manner.[9] The classification describes two types of disorders in HRM: disorders of EGJ outflow and disorders of peristalsis. The key HRM metrics used in CCv4.0 are integrated relaxation pressure (IRP) for assessment of deglutitive relaxation across the LES, distal contractile integral (DCI) for measurement of vigour of oesophageal body contraction, contractile wavefront integrity at 20 mmHg isobaric contour and distal latency (DL) for assessment of latency of deglutitive inhibition. The aim of our retrospective study was to analyse the patterns of oesophageal topographic metrics in patients presenting with symptoms of GERD, refractory to treatment.
MATERIALS AND METHODS
A retrospective analysis was done at a tertiary health-care centre for 30 patients who had presented to the gastroenterology department with clinical features of gastroesophageal reflux and was further referred for oesophageal manometry (HRM). The patients had refractory heartburn and/or regurgitation (no response to a double dose of proton pump inhibitors [PPIs] given for 8 weeks) of at least 6 months duration. Patients with symptoms of dyspepsia (epigastric pain, early satiety and bothersome postprandial fullness), chronic systemic diseases (diabetes, hypertension and asthma) and a history of abdominal surgery were excluded from the study. pH metry data and DeMeester score were included for all patients.
All patients had undergone HRM after 12 h of fasting. A high-resolution 16 channel water perfused gastrointestinal manometric assembly (Trace 2005, Royal Melbourne Hospital, Australia) was used to record LES pressures, peristaltic wave morphology and vigour. The pressure offsets of the water-perfused channels were referenced to atmospheric and hydrostatic pressures by underwater immersion. The oesophageal pressures were recorded with the intra-gastric pressure as baseline.
A real-time picture of pressure topography for each swallow from the upper oesophageal sphincter to LES was generated. Basal LES pressure (BLESP) was measured, and 10 swallows of water, each in supine and upright positions, were assessed for Type of EGJ, IRP, DCI, DL and type of peristaltic wave. CCv4.0 was used to classify the oesophageal peristalsis during analysis. Two independent researchers did a detailed analysis of oesophageal peristaltic waves for each patient and consistently abnormal findings were taken as a positive result.
Statistical analysis was done using the statistical software IBM Statistical Package for the Social Sciences version 20 (IBM Corp Armonk NY). Descriptive statistics such as percentage, mean, and standard deviation (SD) were reported wherever applicable. Karl-Pearson’s correlation was used to determine the correlation between age, BLESP and IRP. Logistic regression was done to determine the presence or absence of motility disorders from age, BLESP and IRP using the enter method with overall-model fit. The area under the curve was calculated from the predicted probabilities of the receiver operating characteristics (ROC) curve. The results were taken as significant when P <0.05.
RESULTS
The data from 30 patients (14 females and 16 males) were analysed. The mean (±SD) age was 49.1 (±13.3) years. Duration of GERD symptoms ranged from 6 months to 3 years with a mean (±SD) of 14.9 (±8.2) months. Twelve patients had presented with symptoms of heart burn, another seven had regurgitation, while the rest 11 patients had both symptoms, all refractory to double doses of PPIs given for at least 8 weeks. A DeMeester score of <14.7 was considered normal in pH metry. The normal range of values in HRM was based on CCv4.0 and is as follows: Normal BLESP in adults - 10-30 mmHg, normal IRP in supine position ≤ 15 mmHg, IRP in upright position ≤ 12 mmHg, DL ≥ 4.5 s and DCI - 450–8000 mmHg.s.cm.
The final diagnosis based on the features of all swallows in each patient is depicted in Figure 1. Our study found 14 patients with normal motility and LES pressure, seven patients with hypotensive LES but no defect in peristaltic wave and nine patients with ineffective oesophageal motility (IEM) (two with hypotensive LES and seven without hypotensive LES). Detailed analysis of patients diagnosed with IEM (n = 9) is shown in Table 1.
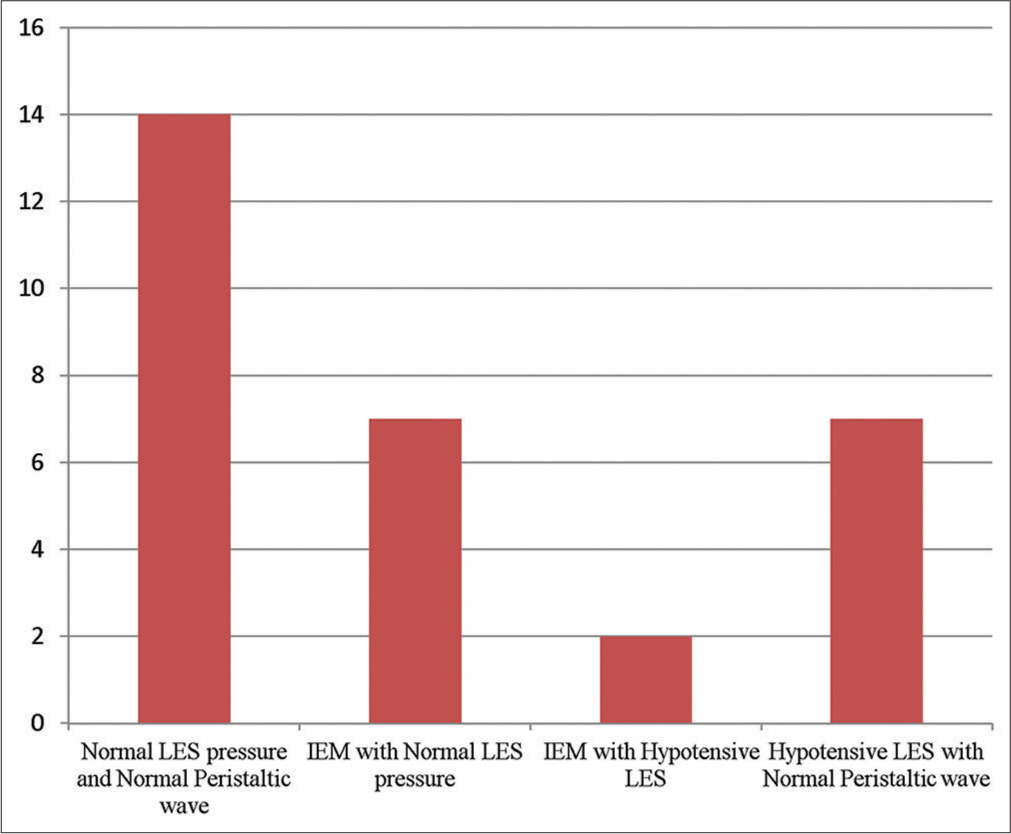
- The diagnosis of patients derived on high-resolution manometry in patients with refractory gastroesophageal reflux disease (n = 30). IEM: Ineffective oesophageal motility, LES: Lower oesophageal sphincter.
| Age years | pH metry score | Type of EGJ | BLES mmHg | IRP-S mmHg | IRP-U mmHg | DL second | DCI mmHg.s.cm | % N-P | % W-P | % Fr-P | % Fa-P | Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 40 y | 36.8 | I | 11.0 | 4.3 | 2.6 | 6.0 | 363 | 30 | 50 | 10 | 10 | IEM |
| 60 y | 50.2 | II | 14.5 | 6.6 | 7.0 | 4.9 | 590 | 20 | 20 | 45 | 15 | IEM |
| 45 y | 39.1 | I | 13 | 7.1 | 3.6 | 4.7 | 100 | 15 | 35 | 10 | 40 | IEM |
| 53 y | 44.2 | II | 15.2 | 2 | 4.5 | 4.8 | 572 | 20 | 25 | 50 | 5 | IEM |
| 51 y | 24.1 | I | 14.1 | 3.0 | 4.0 | 5.1 | 197 | 10 | 40 | 50 | 0 | IEM |
| 35 y | 30.3 | I | 11.8 | 6.7 | 11.0 | 5.0 | 331 | 20 | 5 | 65 | 10 | IEM |
| 35 y | 19.8 | I | 11.0 | 1.8 | 2.8 | 4.8 | 230 | 5 | 35 | 35 | 25 | IEM |
| 70 y | 44.3 | II | 8.3* | 5.1 | 5.5 | 4.6 | 122 | 10 | 55 | 0 | 35 | IEM with hypotensive LES |
| 32 y | 11.2 | II | 3.0* | 1.8 | 2.0 | 4.6 | 1193 | 30 | 5 | 60 | 5 | IEM with hypotensive LES |
Detailed analysis of patients diagnosed with hypotensive LES and normal peristaltic wave (n = 7) is shown in Table 2.
| Age years | pH metry score | Type of EGJ | BLES mmHg | IRP-S mmHg | IRP-U mmHg | DL second | DCI mmHg.s.cm | % N-P | % W-P | % Fr-P | % Fa-P | Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 42 y | 75.5 | I | 4.5* | 1.8 | 3.3 | 5.7 | 909 | 50 | 15 | 35 | 0 | Hypotensive LES with normal peristalsis |
| 45 y | 31.4 | I | 7.9* | 3.1 | 3.5 | 5.2 | 1200 | 75 | 15 | 5 | 5 | Hypotensive LES with normal peristalsis |
| 45 y | 22.3 | II | 2.4* | 2.3 | 2.4 | 5.4 | 507 | 55 | 45 | 0 | 0 | Hypotensive LES with normal peristalsis |
| 39 y | 165.9 | I | 4.5* | 1.5 | 1.7 | 5.2 | 460 | 45 | 30 | 5 | 20 | Hypotensive LES with normal peristalsis |
| 44 y | 30.1 | I | 6.4* | 4.0 | 3.0 | 5.0 | 2385 | 60 | 20 | 20 | 0 | Hypotensive LES with normal peristalsis |
| 70 y | 44.2 | II | 6.9* | 3.7 | 9.5 | 4.9 | 697 | 70 | 0 | 30 | 0 | Hypotensive LES with normal peristalsis |
| 40 y | 24.6 | I | 7.5* | 5.0 | 10.0 | 5.3 | 633 | 65 | 5 | 25 | 5 | Hypotensive LES with normal peristalsis |
The manometric representation of normal oesophageal peristalsis with normal BLESP and a hypotensive LES is shown in Figure 2. Manometric features of a fragmented swallow on the left hand side and failed peristalsis on the right hand side is shown in Figure 3.
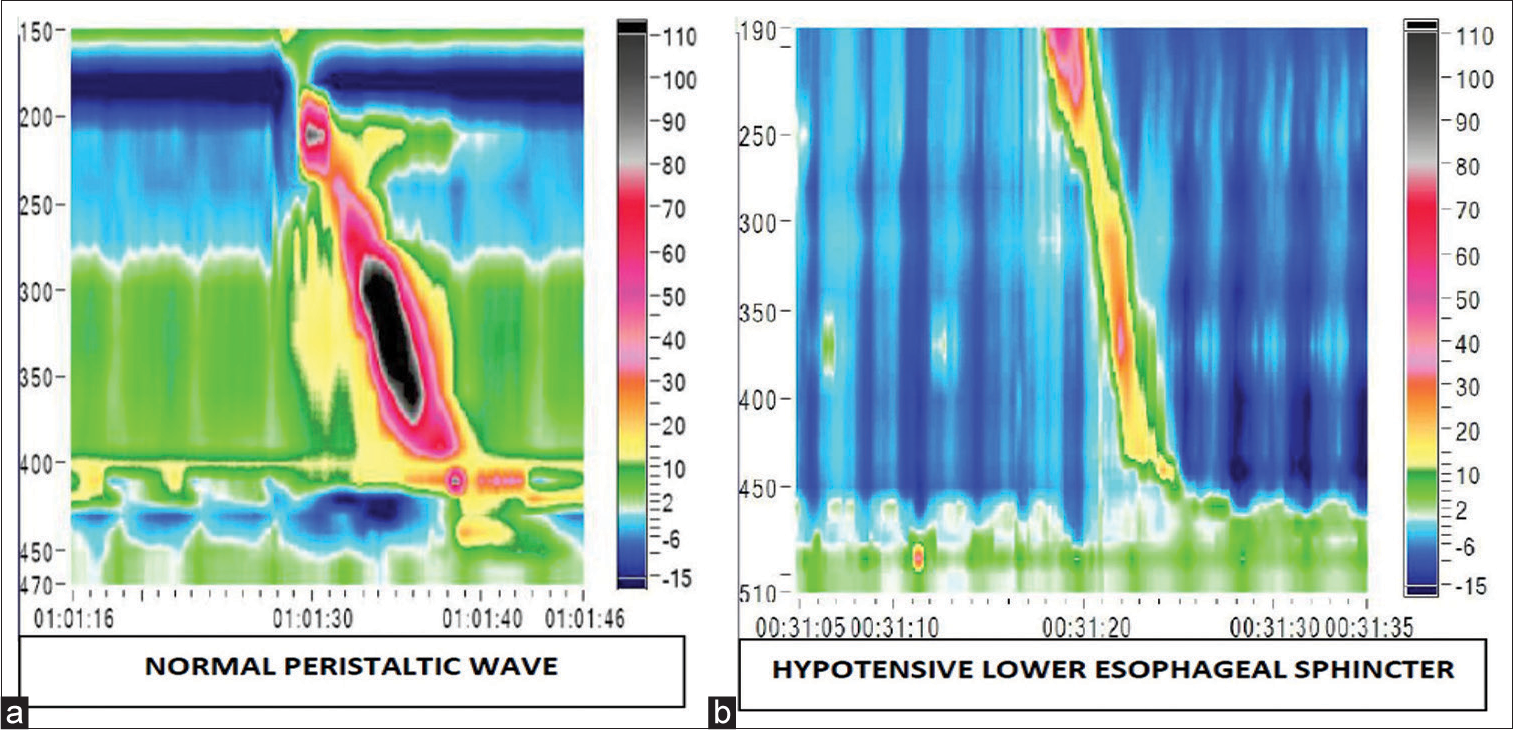
- The colour plot obtained on high-resolution manometry depicts colour coded pressure topography during an oesophageal swallow. (a) The colour plot on the left is of a normal peristalsis, (b) while the one on the right shows hypotensive lower oesophageal sphincter.
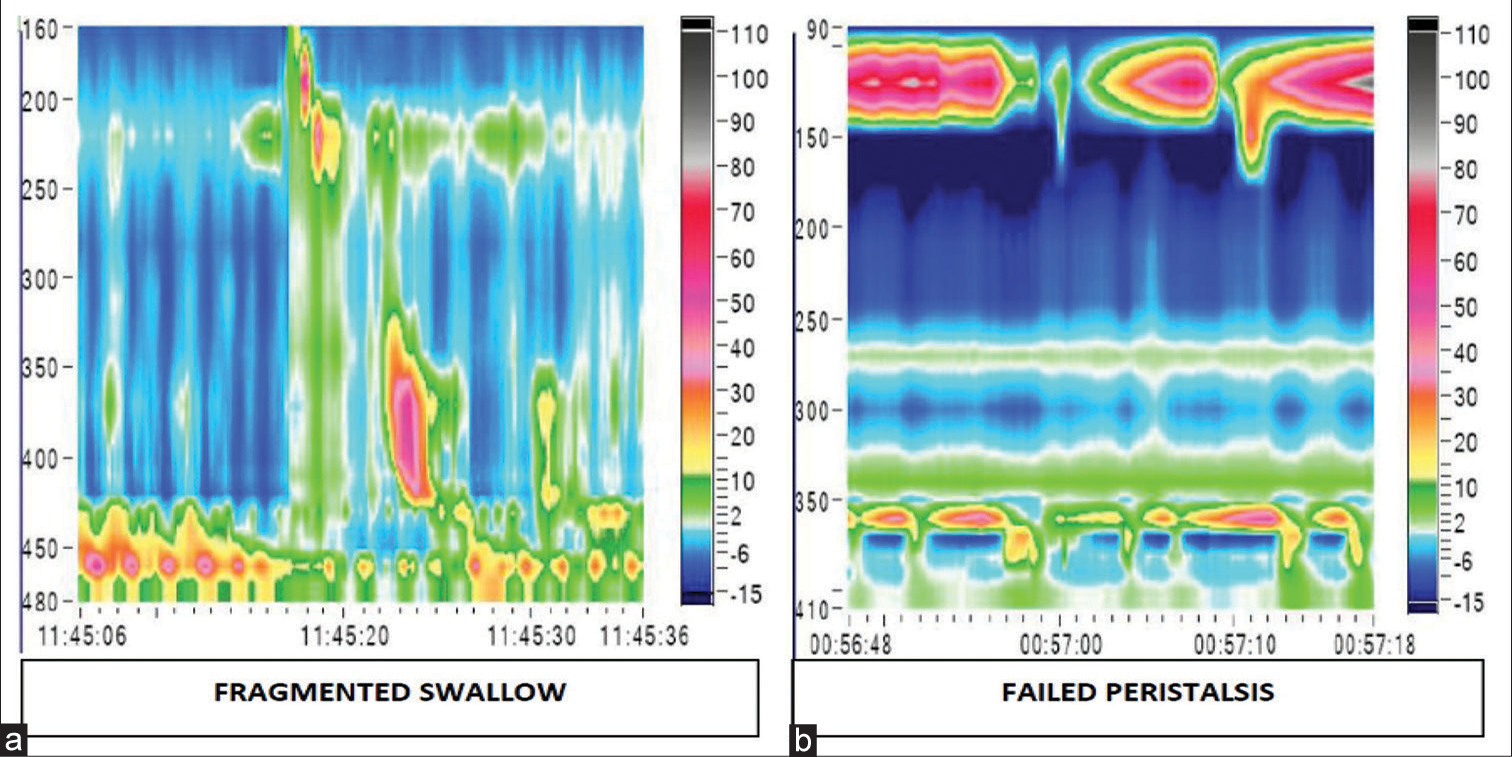
- High-resolution manometry picture obtained in patients with oesophageal motility disorders in gastroesophageal reflux disease. (a) The colour plot on the left shows a fragmented swallow and (b) the one on the right shows failed peristalsis.
The correlation between age and BLESP and between age and IRP were statistically insignificant. Logistic regression for the absence or presence of motility disorders in the final diagnosis when predicted from age (odds ratio [OR] 1.05), BLESP (OR 1.03) and IRP (OR 0.97) did not reveal any significant relation (Chi-square = 3.721, degrees of freedom = 3, P = 0.3). However, the area under the curve for ROC for predicted probabilities of the logistic regression was 0.66 (95% confidence interval 0.47–0.82).
DISCUSSION
GERD consists of a spectrum of diseases which can be endoscopically recognised as NERD, ERD and Barrett’s oesophagus (BE).[10] However, a large group of patients present with symptoms of reflux/heartburn despite normal endoscopy.[6] Manometrically-detectable motility disorders may be associated with oesophageal reflux, with IEM being recognised as an important causative factor of GERD. IEM causes impaired oesophageal clearance, thereby causing increased exposure of the oesophageal mucosa to acid.[11] Our study found 30% of patients with IEM. With the use of HRM, it is possible to clearly delineate specific defects in peristaltic waves, wherein it may be associated with GERD.[8]
Impaired oesophageal clearance may play a crucial role in the pathogenesis of GERD. When the motility is decreased, oesophageal clearance gets affected, which may lead to the development of GERD. Studies in patients with scleroderma, who showed weak/failed peristalsis on HRM, reported that these patients are often affected by GERD.[12,13] A low BLESP is an important factor causing GERD because an ineffective barrier function of the LES leads to exposure of the oesophagus to gastric reflux. About one-fourth of the patients in our study had a hypotensive LES with a normal peristaltic wave. A study done by van Hoeij et al. also reported lower BLESP and lower contractile amplitude in GERD patients as compared to healthy controls.[14]
IEM is reported to be associated with GERD by earlier studies.[15] With the advent of HRM, the definition of IEM has become more specific as the distinction between contractile vigour and contractile pattern is made based on DCI. DCI is used for the assessment of the vigour of oesophageal body contraction. DCI < 100 mmHg.s.cm is classified as failed peristalsis, DCI 100–<450 mmHg.s.cm is classified as weak contraction, while a DCI ≥450 mmHg.s.cm and a large defect (>5 cm) is considered fragmented swallow.[9,16] Large defects (>5 cm) in the 20 mmHg isobaric contour seen on HRM recording correlate with incomplete bolus transit at those sites.[17,18] IEM is diagnosed when at least 50% of swallows are failed peristalsis or more than 70% of swallows are weak contraction/fragmented swallow/failed peristalsis. With the use of the Chicago Classification, the treating physician has definitive criteria for diagnosing patients with oesophageal motility disorders, thereby reducing the number of indeterminate diagnoses.[9,19]
The present study found about 46.66% of patients with normal motility and LES pressure, 23.33% of patients with hypotensive LES but no defect in peristaltic wave and 30% of patients with IEM. A similar study by Kaliyaperumal et al. found IEM in 26.7% and low LES in only 11.7% among 66 patients presenting with GERD symptoms. The incidence of IEM in patients with GERD was higher than that of low LES pressure.[20] Another study by Wang et al. analysed the oesophageal motility characteristics of 176 refractory heartburn patients who had endoscopically recognised reflux patterns, wherein the authors reported weak peristaltic swallows to be the most common motility disorder in both ERD and NERD.[21] Some studies have also shown that delayed bolus transit due to oesophageal peristaltic dysfunction was increasingly prevalent with greater severity of GERD symptoms, with delayed bolus transit being maximally reported in ERD and BE.[22,23] A study concluded that an increase in the amount of mucosal damage from the group without esophagitis to BE worsened oesophageal motility. These findings further suggest that the inflammatory process of GERD may extend from the mucosa to the muscular layer, resulting in hypocontractility and compromising the oesophageal motility of many patients.[24]
There were some limitations in the present study. Patient selection was based on clinical features of refractory regurgitation or heartburn and pH metry findings only. However, heartburn and acid regurgitation have a very high specificity for GERD (89% and 95%, respectively).[25] Further, as endoscopic data were not available, we could not classify the patients based on the endoscopic findings of GERD, which would have further graded the patients based on severity. Furthermore, data on barium swallow were not available for the patients.
The degree of impairment of oesophageal clearance is likely a function of the number of weak/failed peristalsis and the number of breaks in the peristaltic wavefront. Impaired contractility in the oesophageal peristaltic wave may reduce the clearance of the refluxate, thus causing GERD. Therefore, HRM may find greater diagnostic use in such patients who are refractory to treatment.
CONCLUSION
IEM was found in about one third of patients of GERD. HRM may be considered as a modality for aiding diagnosis in GERD cases refractory to treatment.
Ethical approval
The Institutional Review Board has waived the ethical approval for this study.
Declaration of patient consent
Patient’s consent is not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- The Montreal definition and classification of gastroesophageal reflux disease: A global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-20.
- [CrossRef] [PubMed] [Google Scholar]
- Pathophysiology of gastroesophageal reflux disease. Gastroenterology. 2018;54:277-88.
- [CrossRef] [PubMed] [Google Scholar]
- Overview of the mechanisms of gastroesophageal reflux. Am J Med. 2001;111(Suppl 8A):174S-7.
- [CrossRef] [PubMed] [Google Scholar]
- The pathophysiology of gastro-esophageal reflux disease-oesophageal manifestations. Aliment Pharmacol Ther. 2004;20(Suppl 9):14-25.
- [CrossRef] [PubMed] [Google Scholar]
- The pathophysiology of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2007;26:149-60.
- [CrossRef] [PubMed] [Google Scholar]
- Relevance of ineffective oesophageal motility during oesophageal acid clearance. Gut. 2003;52:784-90.
- [CrossRef] [PubMed] [Google Scholar]
- High-resolution manometry: An atlas of esophageal motility disorders and findings of GERD using esophageal pressure topography. Thorac Surg Clin. 2011;21:465-75.
- [CrossRef] [PubMed] [Google Scholar]
- Esophageal motility abnormalities in gastroesophageal reflux disease. World J Gastrointest Pharmacol Ther. 2014;5:86-96.
- [CrossRef] [PubMed] [Google Scholar]
- Esophageal motility disorders on high resolution manometry: Chicago Classification Version 4.0. Neurogastroenterol Motil. 2021;33:e14058.
- [Google Scholar]
- The natural history of gastro-esophageal reflux disease: A comprehensive review. Dis Esophagus. 2017;30:1-9.
- [CrossRef] [Google Scholar]
- Relevance of mild ineffective oesophageal motility (IOM) and potential pharmacological reversibility of severe IOM in patients with gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2007;26:1345-54.
- [CrossRef] [PubMed] [Google Scholar]
- Gastrointestinal motility disorder assessment in systemic sclerosis. Rheumatology (Oxford). 2013;52:1095-100.
- [CrossRef] [PubMed] [Google Scholar]
- High resolution esophageal manometry in systemic sclerosis in the Indian population: An observational study. Indian J Rheumatol. 2020;15:106-10.
- [CrossRef] [Google Scholar]
- Predictive value of routine esophageal high-resolution manometry for gastro-esophageal reflux disease. Neurogastroenterol Motil. 2015;27:963-70.
- [CrossRef] [PubMed] [Google Scholar]
- Ineffective esophageal motility (IEM): The primary finding in patients with nonspecific esophageal motility disorder. Dig Dis Sci. 1997;42:1859-65.
- [CrossRef] [PubMed] [Google Scholar]
- Understanding the Chicago classification: From tracings to patients. J Neurogastroentrol Motil. 2017;23:487-94.
- [CrossRef] [PubMed] [Google Scholar]
- Weak peristalsis in esophageal pressure topography: Classification and association with dysphagia. Am J Gastroenterol. 2011;106:349-56.
- [CrossRef] [PubMed] [Google Scholar]
- Physiology of the oesophageal transition zone in the presence of chronic bolus retention: Studies using concurrent high resolution manometry and digital fluoroscopy. Neurogastroenterol Motil. 2008;20:750-9.
- [CrossRef] [PubMed] [Google Scholar]
- Trends in diagnoses after implementation of the Chicago classification for esophageal motility disorders (V3.0) for HRM studies. Dis Esophagus. 2017;30:1-6.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and manometric profile of patients with GERD in a tertiary care hospital. Int J Adv Med. 2017;4:1658-61.
- [CrossRef] [Google Scholar]
- Esophageal motility characteristics of refractory heartburn: A study based on high resolution manometry and 24 hour pH-impedance monitoring. Chin Med J (Taipei). 2014;94:2650-5.
- [Google Scholar]
- Distinct clinical characteristics between patients with nonerosive reflux disease and those with reflux esophagitis. Clin Gastroenterol Hepatol. 2007;5:690-5.
- [CrossRef] [PubMed] [Google Scholar]
- Oesophageal motility and bolus transit abnormalities increase in parallel with the severity of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2011;34:476-86.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of gastroesophageal reflux on esophageal motility. Rom J Intern Med. 2012;50:233-9.
- [Google Scholar]
- Diagnosis and treatment of Gastroesophageal reflux disease. World J Gastrointest Pharmacol Ther. 2014;5:105-12.
- [CrossRef] [PubMed] [Google Scholar]







