Translate this page into:
Perivascular histamine receptors mediate vasosensory reflex responses elicited by thermal nociceptive stimuli in anaesthetised rat models
*Corresponding author: Sanjeev K. Singh, Department of Physiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India. drssks@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Revand R, Singh SK. Perivascular histamine receptors mediate vasosensory reflex responses elicited by thermal nociceptive stimuli in anaesthetised rat models. Indian J Physiol Pharmacol 2021;65:146-52.
Abstract
Objectives:
Reflex responses elicited by intra-arterial (i.a) instillation of nociceptive agents are known as vasosensory reflex responses. The present study was designed to demonstrate the vasosensory reflex responses evoked by thermal nociceptive stimuli in anaesthetised rat models and to examine the role of perivascular histamine receptors in mediating these responses.
Materials and Methods:
In this study, saline at different temperatures (0°C/30°C/55°C) was instilled retrogradely into the femoral artery to stimulate the perivascular nociceptive terminals and the respiratory movements, BP and ECG were recorded for 10 min. Experiments were performed in four groups of rats. Two groups were dedicated to study the temperature-induced reflex cardiorespiratory (CVR) responses after i.a instillation of cold (0°C) and warm saline (55°C). The responses in these groups were compared with the responses after instillation of normal saline at room temperature (30°C) in a separate group of rats that served as time matched control group. Another group of rats was pre-treated with pheniramine and the responses elicited by warm saline were studied.
Results:
Instillation of warm saline produced immediate (2–6 s) hyperventilatory, hypotensive and bradycardiac responses which were short-lived, while equivolume of normal saline at room temperature did not. Cold saline also elicited the CVR changes of similar quality as that of warm saline but of lesser quantity which were not significantly different from the control group. Pre-treatment with pheniramine significantly attenuated the warm saline-induced reflex responses.
Conclusion:
Activation of perivascular sensory nerve terminals by thermal nociceptive stimuli elicits vasosensory reflex responses altering CVR parameters. Perivascular histamine receptors play a significant role in mediating the temperature-induced vasosensory reflex responses.
Keywords
Nociception
Vasosensory reflex
Histamine receptor
Transient receptor potential channel
Temperature
INTRODUCTION
Chemical mediators of inflammation such as histamine, serotonin, bradykinin, substance P and ATP play a significant role in the pathogenesis of diseases affecting the blood vessels, namely migraine, myocardial infarction, peripheral vascular diseases and atherosclerosis.[1] The contribution of inflammatory mediators (algogens) present in peripheral blood vessels in the reflex modulation of the cardiorespiratory (CVR) system has been described by several workers.[2-4] Reflex CVR alterations produced after intra-arterial (i.a) instillation of algogens into a local segment of medium-sized peripheral blood vessels are known as ‘vasosensory reflex responses.’[2,3] The walls of blood vessels particularly arteries are richly innervated by sensory nerve terminals.[5] Transient receptor potential (TRP) cation channels particularly the Vanilloid receptor-1 subfamily are located in the perivascular nociceptive terminals.[6-8] These afferent nerves when activated by nociceptive chemical, thermal and mechanical stimuli convey the information about the noxious damages in the vicinity to nuclei located in the pons and medulla, which modulate the CVR parameters by reflex mechanisms.[5]
Perivascular nociceptive afferents are known to be activated by a variety of chemical stimuli such as capsaicin, resiniferatoxin, metabolites of polyunsaturated fatty acids and noxious heat.[9-12] The vasosensory reflex responses evoked by chemical agents such as capsaicin, anandamide, ab Me-ATP and bradykinin have been described elsewhere.[2,3,13] Works from our laboratory have demonstrated similar reflex responses using histamine, bradykinin and Mesobuthus tamulus (BT) venom as nociceptive tools.[4,14-18] Although vasosensory reflex responses elicited by chemical nociceptive stimuli have been studied extensively, the same is not true with thermal and mechanical nociceptive stimuli. Therefore, the present study was conducted to study the nature of vasosensory reflex responses elicited by thermal nociceptive stimuli (warm and cold) and to evaluate the role of histamine, a principal mediator of inflammation in mediating these responses. Further, the novel experimental design used in this study facilitated the deposition of nociceptive agent in the local intravascular compartment of femoral artery, thereby stimulating the perivascular afferents with precision.[18]
MATERIALS AND METHODS
The Animal Ethical Committee of our Institute approved the present study (Ref. No. Dean/2019/IAEC/1627 dated 17.11.2019). All animals were handled in accordance with the U.K. Animals (Scientific Procedures) Act, 1986 and EU Directive 2010/63/EU for animal experiments. Chemicals (saline/pheniramine) were instilled into the femoral artery retrogradely through cannula evolved in our laboratory and the volume of injectable was kept minimal (0.1 ml). Six animals were used in each group and the laboratory temperature was maintained at 30°C. At the end of experiments, animals were sacrificed by overdose of anaesthesia. The detailed methodology of dissection, cannulation and recording was standardised, validated and published in our previous articles[4,18] and a relevant description is given below.
Animals and anaesthesia
Adult male rats (Charles-Foster strain; 220.35 g ± 10.27 g) were procured from the Institute Animal House and were kept at 12:12 h light/dark cycle under controlled environment for at least 1 week before the experiments. Food/water was provided to the animals ad libitum. Urethane (Merck, Germany) was freshly dissolved in double-distilled water in the concentration of 0.5 g/ml and was injected to the rats intraperitoneally (1.5 g/kg).
Dissection and recording
Under adequate anaesthesia, tracheostomy was performed to keep the respiratory tract patent. The right femoral triangle was dissected and the femoral artery was cannulated using a 24G, double ported polyethylene cannula filled with freshly prepared heparinised saline (20 IU/ml). The horizontal port of the cannula was connected to the blood pressure (BP) transducer (AD Instruments, Australia) and the vertical port was used to instil the chemicals into the femoral artery.[18] The respiratory movements were recorded by securing the skin over xiphisternum using a suture needle and connecting it to a force transducer (AD Instruments, Australia) through a thread. The electrocardiographic (ECG) potentials were recorded using needle electrodes, connected in standard limb lead-II configuration. At the end of experiments, a known volume (1 ml) of air was injected into the quiescent lung of sacrificed animal through the tracheal tube and the corresponding deflection was computed as ‘x.’ The average amplitude of respiratory excursions for a period of 5 s was measured as ‘h.’ For calculation of respiratory minute volume (RMV), the height (in mm) of respiration was converted to volume (in ml) using the formula (h/x) and was multiplied with the respiratory frequency. The mean arterial pressure (MAP) and heart rate (HR) were computed from the original recordings of the experiments.[18]
Experimental protocol
After dissection and cannulation, 15 min were given to the animals for the stabilisation of CVR parameters. Then, the initial recordings of respiration, BP and ECG were performed. Saline (0.9%, 0.1 ml) at different temperatures (0°C/30°C/55°C) was instilled into the femoral artery and the recordings of the CVR parameters were made for 10 min. One group of rats (n = 6) was devoted to each temperature. The responses with normal saline at room temperature (30°C) served as control responses for the other groups.
In a separate group of animals, the initial recordings of respiration, BP and ECG were performed as mentioned above. Then, pheniramine was instilled (i.a., 0.1 ml, 0.4 mg/kg) and 20 min interval was given for the optimal action of the drug. Subsequently, warm saline was instilled to elicit vasosensory reflex responses and the CVR parameters were recorded for 10 min.
Statistical analysis
The results were presented as mean ± SEM values. The statistical significance between the groups were analysed by comparing the RMV, MAP and HR responses of cold saline and warm saline groups with the normal saline control group. Similarly, the CVR responses with warm saline after pheniramine pre-treatment were compared with the warm saline only group. The comparisons between various groups were made using Student’s t-test for paired/unpaired observations and post hoc correction using Dunnett’s test (two sided) for other observations using SPSS-16.0 software. P < 0.05 was considered statistically significant.
Drugs and instruments
Pheniramine maleate (22.75 mg/ml) was obtained from Sanofi India Ltd., Vadodara, India. Heparin (1000 U/ml) was obtained from Cipla Pharmaceuticals Company, Mumbai. The temperatures of the saline (0°C, 30°C and 55°C) were measured using a laboratory thermometer (Gera Industries Ltd., New Delhi, India) and care was taken to keep the time interval between the measurement of temperature and instillation of saline into the femoral artery <2 s so that only a minimal dissipation of temperature is permitted.
RESULTS
Instillation (i.a) of warm saline produced immediate (2–6 s) hyperventilatory, hypotensive and bradycardiac responses in contrast with equivolume of normal saline at room temperature (30°C). Cold saline produced similar pattern of CVR changes like warm saline but of lesser magnitude, which were not significantly different as compared to the normal saline [Table 1; Figures 1 and 2]. Since warm saline produced optimal responses on all CVR parameters, it was used for the elicitation of vasosensory reflex responses in the subsequent sets of experiments. CVR parameters were recorded for 10 min after instillation of saline but it was found that no significant changes in the CVR parameters took place after 60 s of instillation.
| CVR parameters | Normal saline (30°C) | Cold saline (0°C) | Warm saline (55°C) | Warm saline (55°C) after pheniramine pre-treatment | ||||
|---|---|---|---|---|---|---|---|---|
| Initial | Response | Initial | Response | Initial | Response | Initial | Response | |
| RMV (ml/min) |
191.60±4.36 | 211.07±5.14 | 185.72±5.49 | 236.70±9.63 | 192.55±9.67 | 364.33±20.13* | 199.35±6.16 | 247.15±13.26# |
| MAP (mm Hg) |
91.33±3.12 | 86.00±4.60 | 90.50±7.42 | 76.00±7.50 | 92.17±6.12 | 68.33±6.47* | 103.50±5.10 | 96.83±4.53# |
| HR (bpm) | 282.50±6.89 | 284.83±7.61 | 294.00±7.28 | 278.00±10.36 | 287.17±6.00 | 217.67±23.35* | 295.33±17.53 | 288.83±18.30# |
CVR: Cardiorespiratory, RMV: Respiratory minute volume; MAP: Mean arterial pressure; HR: Heart rate; s: Seconds; bpm: Beats per min. An (*) indicates significant difference in the CVR parameters as compared with the normal saline (30°C) control group (P<0.05, posthoc Dunnett’s test) and with the corresponding initial values for warm saline (P<0.05, paired t-test). An (#) indicates significant attenuation in the CVR changes as compared to the warm saline only group (P<0.05, post hoc Dunnett’s test)

- Original recordings showing the effect of intra-arterial instillation of cold saline (0°C) and warm saline (55°C) on respiration (Resp), blood pressure (BP) and electrocardiogram (ECG) as compared with the effects of normal saline at room temperature (30°C). The recordings depict 10 s before and 40 s after instillation of saline. The time scale is shown in the lower panel. Dotted lines indicate the point of instillation of saline.

- Time-response plots showing the effect of intra-arterial instillation cold (0°C) and warm saline (55°C) on respiratory minute volume (RMV), mean arterial pressure (MAP) and heart rate (HR). The control responses after instillation normal saline at room temperature (30°C) are given in each row along with cold saline responses. The values are in mean±SEM from six experiments. An asterisk (*) indicates significant difference in the responses as compared to normal saline control group (P<0.05, post hoc Dunnett’s test). Arrows indicate the point of instillation of saline.
CVR alterations after i.a instillation of warm saline
Instillation of warm saline produced an immediate increase in the RMV and decrease in the MAP and HR parameters. The changes in the CVR parameters with warm saline were significantly different as compared with normal saline at room temperature [Table 1; Figures 1 and 2; P < 0.05, post hoc Dunnett’s test] and with the corresponding initial values with warm saline [Table 1; Figures 1 and 2; P < 0.05, paired t-test]. The responses were short lived and returned back to the initial values within 30 s and remained at that level for the entire period of observation.
CVR alterations after i.a instillation of cold saline
Instillation of cold saline also produced an immediate increase in the RMV and decrease in the MAP and HR parameters. The changes in the CVR parameters with cold saline were not significantly different as compared with normal saline at room temperature [Table 1; Figures 1 and 2; P > 0.05, post hoc Dunnett’s test]. Like warm saline, the responses with cold saline were short lived and returned back to the initial level within 30 s and remained at that level for the remaining period.
Latencies of the CVR responses
The duration of onset of responses (latency) was different for different CVR parameters with different temperatures of saline [Figure 3]. Latency was shortest for HR and longest for MAP responses. The latencies for hypotensive responses were 5.83 ± 0.28 s and 5.50 ± 0.55 s for warm and cold saline, respectively. The latencies for hyperventilatory responses were 2.17 ± 0.17 s and 2.17 ± 0.11 s for warm and cold saline, respectively. The latencies for bradycardiac changes were 1.42 ± 0.20 s and 1.25 ± 0.17 s for warm and cold saline, respectively. It was observed that the difference in the latencies of MAP, RMV and HR responses with warm saline was not statistically significant from the cold saline group [Figure 3; P > 0.05, unpaired t-test] but the latencies of MAP responses were significantly different as compared with the latencies of RMV and HR responses [Figure 3; P < 0.05, unpaired t-test]. Normal saline at room temperature did not produce any significant response on the CVR parameters so the latency cannot be calculated for this group.
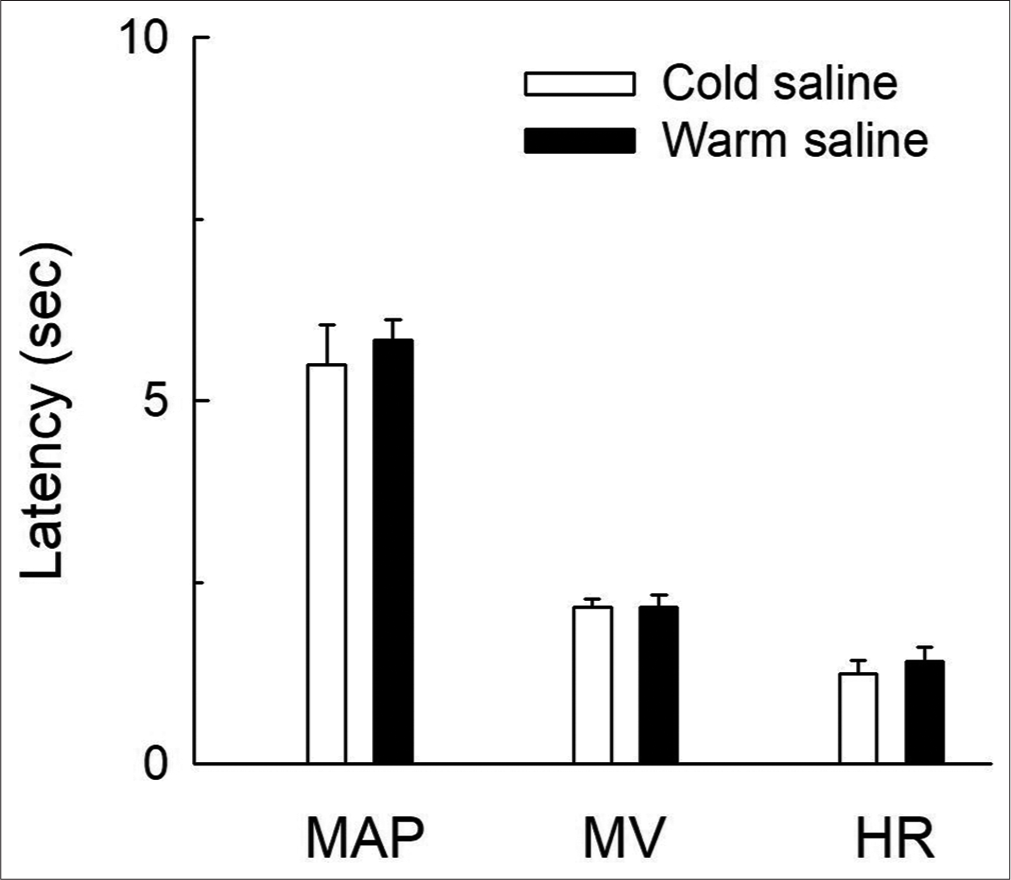
- Bar diagrams showing the latencies of mean arterial pressure (MAP), respiratory minute volume (RMV) and heart rate (HR) responses for cold (0°C) and warm saline (55°C). The values are expressed as mean±SEM from six experiments. The latency of MAP responses is significantly different as compared to the RMV and HR responses (P<0.05, unpaired t-test).
Pheniramine pre-treatment attenuated warm saline-induced CVR changes
Instillation (i.a.) of pheniramine per se did not produce any change in the resting RMV, MAP and HR. However, pheniramine pre-treatment attenuated the warm saline-induced hyperventilatory responses (from 89% to 24% of initial value), hypotensive responses (from 26% to 6% of initial value) and bradycardiac responses (from 24% to 3% of initial value) significantly [Table 1; Figure 4; P < 0.05, post hoc Dunnett’s test].

- Pheniramine pre-treatment (+Phen) blocked the warm saline (WS)-induced responses. The time-response plots showing the effect of intra-arterial instillation of WS on respiratory minute volume (RMV), mean arterial pressure (MAP) and heart rate (HR) in pheniramine pre-treated (+Phen +WS) group and WS (55°C) only group are shown in (a-c). An asterisk (*) indicates significant difference in the CVR responses in +Phen +WS group as compared to the WS only group (P<0.05, post hoc Dunnett’s test). Arrows indicate point of instillation of saline. (d) The original recordings showing the effect of WS-induced changes on respiration (Resp), blood pressure (BP) and electrocardiogram (ECG) in pheniramine pre-treated animals. Dotted line indicates the point of injection of WS after +Phen.
DISCUSSION
The present observations demonstrate that instillation of warm saline into a segment of femoral artery elicits immediate hyperventilatory, hypotensive and bradycardiac changes of shorter latency, as compared with equivolume of normal saline at room temperature, excluding the possibilities of stretch/ischemia-induced responses on the arterial wall. Cold saline also produces similar pattern of CVR changes as that of warm saline but of lesser magnitude which is not significantly different from the control group. All the responses are short-lived and CVR parameters return back to the initial level within 30 s after instillation of nociceptive agents. Pheniramine pre-treatment significantly attenuates warm saline-induced reflex CVR alterations.
Studies elsewhere showed that around 45% of nociceptors (TRPV-1) are activated by temperatures of >45°C and around 60% of them are stimulated by temperatures of >52°C.[19-23] Therefore, in our experiments, the temperature of warm saline was kept at 55°C so that adequate thermal stimulus could be delivered to the perivascular nerve endings, as a dissipation of 1–2°C is expected in the process of instillation. Regarding cold, only around 20% of nociceptors (TRPM-8) are activated by temperatures of <4°C.[24-28] Hence, the temperature of cold saline in our experiments was chosen at 0°C. Utmost care was taken at every step to minimise the temperature dissipation, by ensuring that the saline is instilled into femoral artery within 2 s of measurement of its temperature. Further, temporary alterations in the properties of receptor proteins are expected after exposure to higher temperatures while such alterations are known to be minimum with colder temperatures.[29,30] Hence, very high temperatures (>60°C) were avoided in this study as it may permanently denature the perivascular receptors.
Workers in the past have shown CVR alterations elicited by intravascular injection of various chemical nociceptive agents such as capsaicin, ATP, anandamide, cinnamaldehyde, acrolein, histamine and bradykinin.[2-4,13,18,31,32] Earlier works from our laboratory have also shown the reflex responses elicited by i.a instillation of Indian red scorpion venom as nociceptive agonist.[14-17] In the present study, the vasosensory reflex responses are elicited by activating perivascular afferent neurons using thermal nociceptive stimuli. Further, in this study, a 24G double ported cannulation of femoral artery is performed to instil the nociceptive agonist and to record the BP at the same time.[18] This minimises the distress to the animal by eliminating the need for separate carotid artery cannulation for BP recording as performed earlier. The cannula also facilitates the deposition of the nociceptive agents in a local segment of femoral artery, thereby eliciting vasosensory reflex responses more precisely.
The shorter latencies of responses show that the CVR changes observed in the present study are reflex mediated responses as humoral responses cannot exhibit so quickly. The perivascular nociceptive afferents contain different nerve fibre types, namely fast Aq and slow C fibres. The different latencies of RMV, MAP and HR parameters support the above ideology and theoretically suggest the possible existence of different sets of afferents of different nerve fibre types, for different CVR parameters. In this study, it can be observed that MV, MAP and HR responses elicited by warm saline were significantly attenuated after pheniramine pre-treatment. Histamine receptors (H1 and H2) are already known to be located on the vascular endothelium. Works elsewhere have demonstrated that activation of histamine receptors potentiates and even cross-activates vascular TRP receptors and vice versa.[11,25,33-35] This cross-activation between perivascular receptors is a major contributor to the nociceptive responses elicited by chemical mediators of inflammation in health and diseases. The attenuation of warm saline-induced responses by pheniramine in our experiments points out the role of perivascular histamine receptors in mediating vasosensory reflex responses elicited by thermal nociceptive stimuli. However, interaction of receptors at the molecular level and pathways responsible for the reflex CVR changes needs to be explored further.
CONCLUSION
Thermal stimuli of appropriate strengths elicit vasosensory reflex responses altering CVR parameters that is, RMV, MAP and HR. Pheniramine, a non-selective histamine receptor antagonist, attenuates these responses indicating the role of perivascular histamine receptors in producing the temperature-induced responses. The activation of perivascular nociceptors by thermal stimuli demonstrates the modulation of CVR parameters due to the local changes in the blood temperatures within peripheral vascular compartments (e.g. blood vessels adjacent to the exercising muscles) in contrast to the systemic temperature changes as occurring in fever and hyperthermia. Our study thus supports the hypothesis regarding the involvement of medium-sized peripheral blood vessels in the reflex modulation of the CVR system in states of health and diseases.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- The role of histamine in neurogenic inflammation. Br J Pharm. 2013;170:38-45.
- [CrossRef] [PubMed] [Google Scholar]
- Anandamide induces cardiovascular and respiratory reflexes via vasosensory nerves in anesthetized rats. Br J Pharmacol. 2001;134:655-63.
- [CrossRef] [PubMed] [Google Scholar]
- Perivascular nerves induce cardiorespiratory reflexes in response to algogens in anesthetized rats. Neurosci Res. 2004;50:271-81.
- [CrossRef] [PubMed] [Google Scholar]
- Instillation of bradykinin into femoral artery elicits cardiorespiratory reflexes involving perivascular afferents in anesthetized rats. Physiol Int. 2020;107:40-54.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of the effects of intravenously injected capsaicin in the rat. Naunyn Schmiedebergs Arch Pharmacol. 1982;320:54-7.
- [CrossRef] [PubMed] [Google Scholar]
- Capsaicin-induced reflex fall in rat blood pressure is mediated by afferent substance P-containing neurons via a reflex centre in the brainstem. Naunyn Schmiedebergs Arch Pharmacol. 1983;324:293-5.
- [CrossRef] [PubMed] [Google Scholar]
- The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816-24.
- [CrossRef] [PubMed] [Google Scholar]
- C nociceptor activity in human nerve during painful and non-painful skin stimulation. J Neurol Neurosurg Psychiatry. 1981;44:600-7.
- [CrossRef] [PubMed] [Google Scholar]
- Peripheral mechanisms of somatic pain. Anaesthesiology. 1988;68:571-90.
- [CrossRef] [PubMed] [Google Scholar]
- Thermo TRP channels as modular proteins with allosteric gating. Cell Calcium. 2007;42:427-38.
- [CrossRef] [PubMed] [Google Scholar]
- Sensory TRP channels: The key transducers of nociception and pain. Prog Mol Biol Transl Sci. 2015;131:73-118.
- [CrossRef] [PubMed] [Google Scholar]
- Activation of P2X receptors for adenosine triphosphate evokes cardiorespiratory reflexes in anesthetized rats. J Physiol. 1998;359:1-18.
- [CrossRef] [PubMed] [Google Scholar]
- Intra-arterial injection of Mesobuthus tamulus venom elicits cardiorespiratory reflexes involving perivascular afferents. Toxicon. 2005;46:820-6.
- [CrossRef] [PubMed] [Google Scholar]
- Injection of Mesobuthus tamulus venom in distal segment of femoral artery evokes hyperventilatory and hypertensive responses in anesthetised rats. Neurosci Lett. 2008;438:64-6.
- [CrossRef] [PubMed] [Google Scholar]
- Vasosensory responses elicited by Indian red scorpion venom last longer than capsaicin-induced responses. Indian J Exp Biol. 2008;46:755-9.
- [Google Scholar]
- Nociceptive vascular reflexes evoked by scorpion venom modulate cardiorespiratory parameters involving vanilloid receptor 1 in anaesthetised rats. Neurosci Lett. 2009;451:194-8.
- [CrossRef] [PubMed] [Google Scholar]
- Retrograde cannulation of femoral artery: A novel experimental design for precise elicitation of vasosensory reflexes in anesthetized rats. MethodsX. 2020;7:101017.
- [CrossRef] [PubMed] [Google Scholar]
- Inhibition of rapid heat responses in nociceptive primary sensory neurons of rats by vanilloid receptor antagonists. J Neurophysiol. 1999;82:2853-60.
- [CrossRef] [PubMed] [Google Scholar]
- Noxious heat activates all capsaicin-sensitive and also a sub-population of capsaicin-insensitive dorsal root ganglion neurons. Neuroscience. 1999;88:995-7.
- [CrossRef] [Google Scholar]
- TREK-1, a K+ channel involved in polymodal pain perception. EMBO J. 2006;25:2368-76.
- [CrossRef] [PubMed] [Google Scholar]
- Differential effects of TRPV channel block on polymodal activation of rat cutaneous nociceptors in vitro. Exp Brain Res. 2009;196:31-44.
- [CrossRef] [PubMed] [Google Scholar]
- Calcium entry via TRPV1 but not ASICs induces neuropeptide release from sensor neurons. Mol Cell Neurosci. 2014;61:13-22.
- [CrossRef] [PubMed] [Google Scholar]
- "Cold" fibre population innervating palmar and digital skin of the monkey: Responses to cooling pulses. J Neurophysiol. 1973;36:325-46.
- [CrossRef] [PubMed] [Google Scholar]
- Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183-7.
- [CrossRef] [PubMed] [Google Scholar]
- Response properties of mechanoreceptors and nociceptors in mouse glabrous skin: An in vivo study. J Neurophysiol. 2001;85:1561-74.
- [CrossRef] [PubMed] [Google Scholar]
- Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379-86.
- [CrossRef] [PubMed] [Google Scholar]
- The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2008;448:204-8.
- [CrossRef] [PubMed] [Google Scholar]
- Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38:397-421.
- [CrossRef] [Google Scholar]
- TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371-8.
- [CrossRef] [PubMed] [Google Scholar]
- Behavioral evidence of thermal hyperalgesia and mechanical allodynia induced by intradermal cinnamaldehyde in rats. Neurosci Lett. 2010;473:233-6.
- [CrossRef] [PubMed] [Google Scholar]
- Agonist-induced sensitisation of the irritant receptor ion channel TRPA1. J Physiol. 2016;594:6643-60.
- [CrossRef] [PubMed] [Google Scholar]
- The TRPV1/2/3 activator 2-inoethoxydiphenylborate sensitizes native nociceptive neurons to heat in wild type but not TRPV1 deficient mice. Neuroscience. 2015;135:1277-84.
- [CrossRef] [PubMed] [Google Scholar]
- TRPV1 and PLC participate in histamine H4 receptor-induced itch. Neural Plast. 2016;16:1682972.
- [CrossRef] [PubMed] [Google Scholar]
- TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: Pro-inflammatory response induced by their activation and their sensitization. Protein Cell. 2017;8:644-61.
- [CrossRef] [PubMed] [Google Scholar]






