Translate this page into:
Role of nitric oxide in determination of large intestinal contractility in neonatal rats
*Corresponding author: Parul Sharma, Department of Physiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India. drparulsharmajpr@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Singh S, Sharma P, Dixit D, Mandal MB. Role of nitric oxide in determination of large intestinal contractility in neonatal rats. Indian J Physiol Pharmacol. 2024;68:9-17. doi: 10.25259/IJPP_374_2023
Abstract
Objectives:
Nitric oxide (NO) plays a key role in inhibiting the contractility of gut smooth muscles in various species, and NG-nitro-L-arginine methyl ester (L-NAME) is a critical NO synthase inhibitor. Previous research investigating the role of NO in regulating gut motility focused on adult animals. Therefore, more research is needed to determine their status in the gut of newborns. Our study intended to understand how NO impacts the large gut contractility, in vitro, in rats, both neonates and adults, to get a better insight into the physiological role of NO in regulating large gut motility, particularly in neonates.
Materials and Methods:
In an organ bath preparation, the segments of a large gut (colon and rectum) were subjected to various concentrations of nitroglycerin (NG) (0.01–100 mM), a NO donor, cumulatively. In another group, pre-treatment with L-NAME (100 mM) was done to evaluate the blocking effect of NO on the contractile tension and frequency.
Results:
NG induced relaxation in the colon and rectum of adult rats in a similar manner. NG caused significantly greater relaxation in neonates’ rectums than in their colons. In neonatal and adult rats, L-NAME pre-application inhibited NG-induced relaxation in contractile tension. Exposure to different concentrations of NG decreased contractile frequency in adult rats’ colons and rectum. However, L-NAME pre-treatment did not affect the decrease in contractile frequency caused by NG. In neonates, NG caused a concentration-dependent reduction in contractile frequency, and a decrease in contractile frequency in the rectum was more than that in the colon. However, L-NAME pre-treatment did not affect the reduction in contractile frequency caused by NG.
Conclusion:
Nitrergic mechanisms have possibly been present since birth. The intensity of control by NO may be different in the colon and rectum. The differences in NO sensitivity in adults and neonates demonstrated the changes during development.
Keywords
Nitric oxide
L-NAME
Nitroglycerin
Rectum
Colon
INTRODUCTION
The motor function of the gastrointestinal tract (GIT) depends on a fine balance between excitatory and inhibitory neurotransmission in GIT smooth muscle.[1] Major excitatory and inhibitory agents are mediated through cholinergic and adrenergic mechanisms, respectively.[2]
However, other mechanisms called ‘non-adrenergic non-cholinergic (NANC) may also play a crucial role in regulating GIT motility’.[3] Out of various NANC mechanisms, nitric oxide (NO) is one of the most important inhibitory neurotransmitters in the GIT. NO regulates intestinal motility, gastric emptying, and antral motor activity. The production of NO in tissues is facilitated by NO Synthase (NOS) using arginine. There are three isoforms of the NOS enzyme: neuronal (nNOS), inducible (iNOS), and endothelial (eNOS). Of these three, neuronal or nNOS is constitutive, calcium-dependent, and present in the myenteric neurons of the gut smooth muscle. NO mediates its effects directly or by activating soluble guanylyl cyclase (GC-S), which produces the second messenger, cyclic guanosine monophosphate.[3-8]
NO is involved in inhibiting the contractility of GI smooth muscles in various species. For example, in adult animals, NO has been shown to induce relaxation in multiple species across different regions of the small intestine. Furthermore, it was reported that NO decreased contractile response in the small intestine of the rabbit.[8] NO has been known to induce relaxation in the mouse small intestine.[9]
One report[10] showed that NO at a low concentration caused contractions in the longitudinal muscle of the small intestine of rats, followed by relaxation at a high concentration. In addition, reduced spontaneous contractile activity was observed in rabbit ileum,[11] sheep intestine,[12] human gastric fundus strips,[13] and small intestine.[14]
Studies in the large gut revealed NO-mediated relaxation in the human colon[15] and rat colon.[16]
Earlier studies show that NG-nitro-L-arginine methyl ester (L-NAME) is important in preventing NO-induced relaxation in the gastrointestinal segments of various species.[15-17] L-NAME is a known NOS inhibitor.
The previous studies have examined the impact of NO on the gut of adult animals, leaving its effects on the neonatal gut uncertain. This study aims to determine and compare how NO affects the large gut motility in neonatal and adult rats to gain a more thorough insight into its physiological role in regulating the motility of large gut in neonates.
MATERIALS AND METHODS
Both male and female albino rats (Charles foster strain) were used in this study. Adult (4–6 months old) and neonates (10–16 days old) rats were grouped separately. The study was approved by the Institute’s Ethical Clearance Committee (No. Dean/12-13/CAEC/32). Animals were kept in a room with recommended temperature, humidity, and light cycle conditions, and they had access to food and drink as needed.
Adult rats were sacrificed by cervical dislocation and exsanguination. At the same time, decapitation was used to kill newborns. Immediately after sacrificing the animals, a vertical incision was made in the abdomen. A portion of the colon and rectum was dissected and thoroughly cleansed by flushing out the contents. Following this, a sample from the cleaned part was placed in a petri dish that contained a cold Krebs-Ringer solution. The solution was bubbled with 100% oxygen.
The longitudinal segments, about 12–15 mm long, were from the sample and were taken in an organ bath. The organ bath was filled with Krebs-Ringer solution (12 mL). The temperature was maintained at 37 ± 1°C, and the solution was continuously oxygenated (100% O2). The segments were mounted vertically, so one end was attached to a glass tube support and the other to a force transducer (AD Instruments), giving an initial tension of 0.25–0.5 g.
Recording procedure
The recording procedure followed was as described in earlier studies.[18] Briefly, the mounted segments were left to equilibrate for 30 min. The contractions recorded were of the isometric type and were enhanced using a bridge amplifier. The data were digitised with the help of an analogue/digital interface (Power Lab 4/ST system) and acquired on a computer. The recordings were shown and assessed using the Chart-5 for Windows software developed by AD Instruments in Sydney, Australia. Calibration for tension (0–10 g) was done both before and after recording the contractile responses. Once stabilisation was achieved, spontaneous contractions were recorded for 30 min. The gut segments were treated with varying concentrations of nitroglycerin (NG) (ranging from 0.01 to 100 mM), a NO donor. The concentrations were applied cumulatively. To assess the blocking effect of NO on contractility, we used L-NAME (100 mM). After the recording, the tissue segment was taken out of the organ bath, and after removing any extra water, it was immediately put on a blotting paper. The damaged parts were cut off by removing the ends. The weight of wet tissue was noted precisely to determine the contractile response per unit weight of tissue (g/g wet tissue).
Chemicals
The Krebs-Ringer solution was prepared following standard composition and pH.[18]
NO donor NG was purchased from Neon Laboratories, Ltd., Thane, Maharashtra, India. L-NAME was procured from Sigma Aldrich Chemicals Pvt. Ltd., India. The concentration of NG in the injection vial was 4.4037 mM. A 10 mM stock solution of L-NAME hydrochloride was prepared in distilled water. Just before the experiment, dilutions, as required, were prepared in Krebs-Ringer solution.
Experimental protocol
Total 24 rats (12 adults and 12 neonates) were used in the study. There were two experimental groups for each adult and neonate rat. In the first group of experiments, after the initial recordings (baseline/control) from the colon and rectum of adult (n = 4–6) and neonate (n = 4–6) rats, the tissue was exposed to varying cumulative bath concentrations of NG (0.01–100mM). The exposure time for each bath concentration was 10 min. In the second group, after taking initial recordings, the tissue was treated with L-NAME (100 mM, n = 4–6). The exposure time was 10 min. Following this, the segment was exposed to various cumulative concentrations of NG.
Statistical analysis
The contractions were measured in two parameters: contractile tension (g/g wet tissue) and contractile frequency (number of contractions/min). The values of the parameters before the application of varying doses of NG, with or without treatment, were considered 100%. The changes in the parameters were expressed as a percentage of the initial.
The mean ± standard error of the mean values of the percentage of initial highest contractile tension (g/g wet tissue) and frequency (contractions/min) in different groups were calculated. Two-way Analysis of Variance (ANOVA), Post hoc Bonferroni test for multiple comparisons, and Student’s t-test (Graph Pad Prism 4 software) were performed as needed. P < 0.05 was considered significant.
RESULTS
Table 1 shows the effect of L-NAME (100 mM) on contractile tension of spontaneous contractions in colon and rectum of adult and neonate rats. L-NAME pre-treatment significantly increased the contractile tension in all the groups.
| Adult rats (% of initial) | Neonate rats (% of initial) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline contractile tension at 37°C | Contractile tension in L-NAMEa (100 µM) pre-treated group | Baseline contractile tension at 37°C | Contractile tension in L-NAME (100 µM) pre-treated group | ||||
| Colon | Rectum | Colon | Rectum | Colon | Rectum | Colon | Rectum |
| 100±0.0 | 100±0.0 | 122.79±10.40* | 166.94±23.12* | 100±0.0 | 100±0.0 | 116.15±5.75* | 121.86±10.61* |
Effect of NG treatment on contractile tension
In adult colon and rectum
In the adult colon and rectum, contractile tension decreased by 72% and 75%, respectively, at the highest concentration of NG (100 mM) [Figures 1a and b and recording 1]. NG-induced relaxation was similar in the adult colon and rectum (P > 0.05, two-way ANOVA). The EC50 values in the adult colon and rectum were 4.2 mM and 15 mM, respectively.
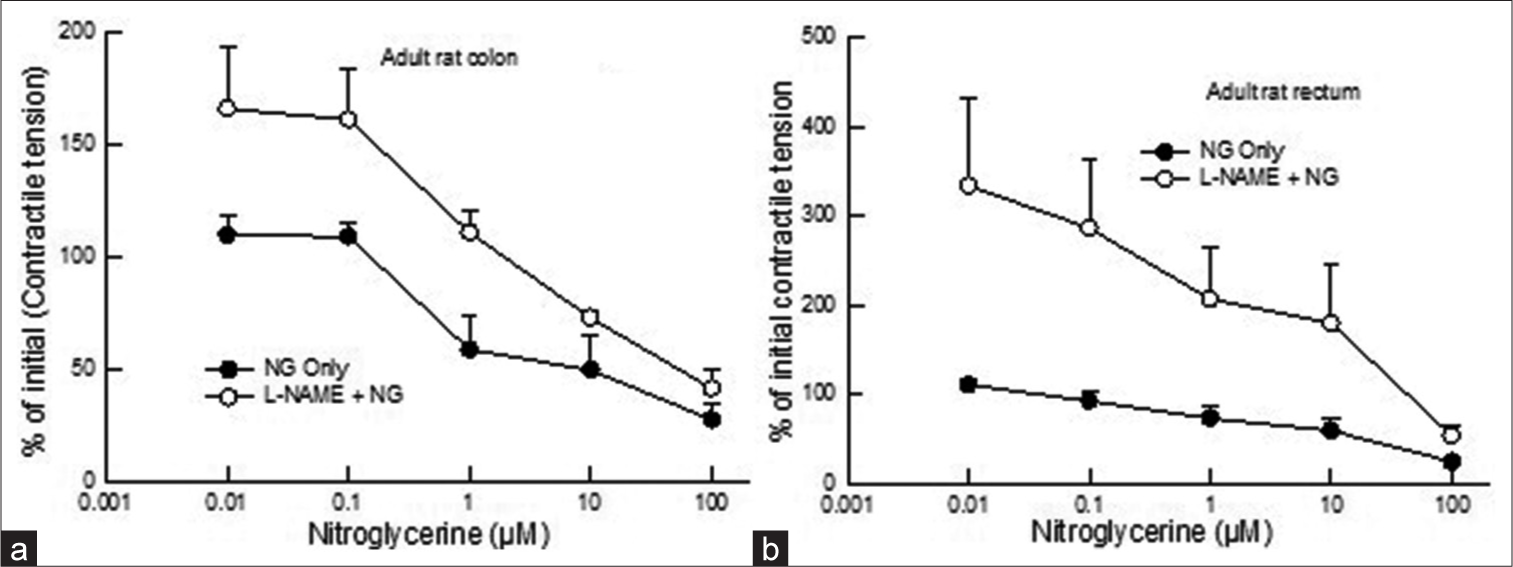
- Dose-response curve showing the comparison of the effect of NG (0.01–100 mM) on contractile tension (% of initial) in adult rat (a) colon and (b) rectum after pre-application of L-NAME (100 mM). Pre-treatment of L-NAME inhibited nitroglycerin-induced decrease in contractile tension (P < 0.05, two-way Analysis of Variance). Data points indicate the mean ± standard error of the mean values. NG: Nitroglycerin, L-NAME: N--nitro-L-arginine methyl ester.
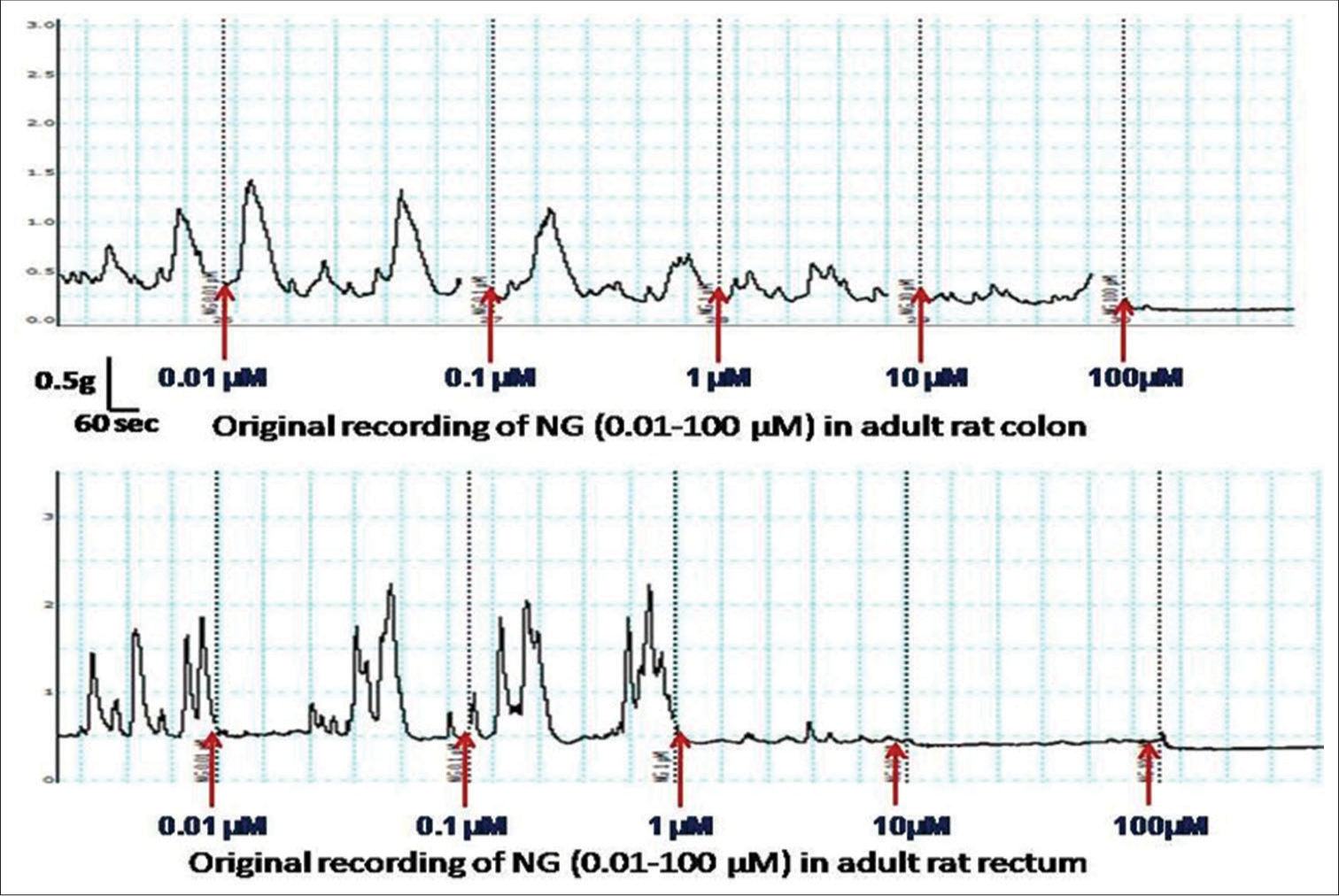
- Image showing an original recording of contractile response at different concentrations of nitroglycerin (0.01–100 mM) in the colon and rectum of adult rats.
In neonatal colon and rectum
There was a 30% and 57% decrease in contractile tension at 100 mM of NG in the neonate rat colon and rectum, respectively [Figures 2a and b and recording 2]. Although the decline was more in the rectum than the colon, these were insignificant when two-way ANOVA was applied (P > 0.05, two-way ANOVA). However, the relaxing effect in the rectum with 10 and 100 mM of NG was significantly more than in the colon (P < 0.05, Student’s unpaired t-test). The EC50 values in the neonate colon and rectum were more than 100 mM and 35 mM, respectively.

- Comparative dose-response curve showing the effect of NG (0.01–100 mM) on contractile tension (% of initial) in neonate rat (a) colon and (b) rectum before and after L-NAME (100 mM) application. Pre-treatment of L-NAME inhibited NG-induced decrease in contractile tension in neonate colon and rectum (P < 0.05, two-way Analysis of Variance). Data points indicate mean ± standard error of the mean values. NG: Nitroglycerin, L-NAME: N--nitro-L-arginine methyl ester.

- Image showing an original recording of contractile response at different concentrations of nitroglycerin (0.01–100 mM) in the colon and rectum of neonate rat.
NG-induced changes in contractile tension after L-NAME (100 mM) pre-treatment
In adult colon and rectum
In the adult rat colon, L-NAME pre-application inhibited NG-induced relaxation in contractile tension (P < 0.05, twoway ANOVA) [Figures 1a and b and recording 3].

- Image showing an original recording of contractile response at different concentrations of nitroglycerin (0.01–100 mM) after L-NAME in the colon of neonate rat. L-NAME: N--nitro-Larginine methyl ester.
In adult rat rectum, pre-exposure of L-NAME prevented a decrease in contractile tension to NG (P < 0.05, twoway ANOVA) and enhanced the contractile activity. The blocking effect of L-NAME was more pronounced at lower concentrations of NG [Figures 1a and b and recording 4].

- Image showing an original recording of contractile response at different concentrations of nitroglycerin (0.01–100 mM) after L-NAME in the rectum of neonate rat. L-NAME: N--nitro-Larginine methyl ester.
In neonatal colon and rectum
In neonate rat colon, L-NAME pre-incubation inhibited relaxation in contractile tension to NG (P < 0.05, two-way ANOVA). The response was more pronounced at lower concentrations (0.01–1 mM) of NG [Figures 2a and b and recording 3].
Similarly, in the neonate rectum, pre-treatment of L-NAME (100 mM) also inhibited NG-induced relaxation significantly (P < 0.05, two-way ANOVA) [Figures 2a and b and recording 4].
Effect of NG on the contractile frequency and NG-induced changes in contractile frequency after L-NAME (100 mM) pre-treatment
In adult colon and rectum
Exposure to varying bath concentrations of NG decreased contractile frequency [Figures 3a and b]. There was no difference between the response of the colon and rectum (P > 0.05, two-way ANOVA). L-NAME pre-treatment did not affect the decrease in contractile frequency to NG (P > 0.05, two-way ANOVA) [Figures 3a and b].
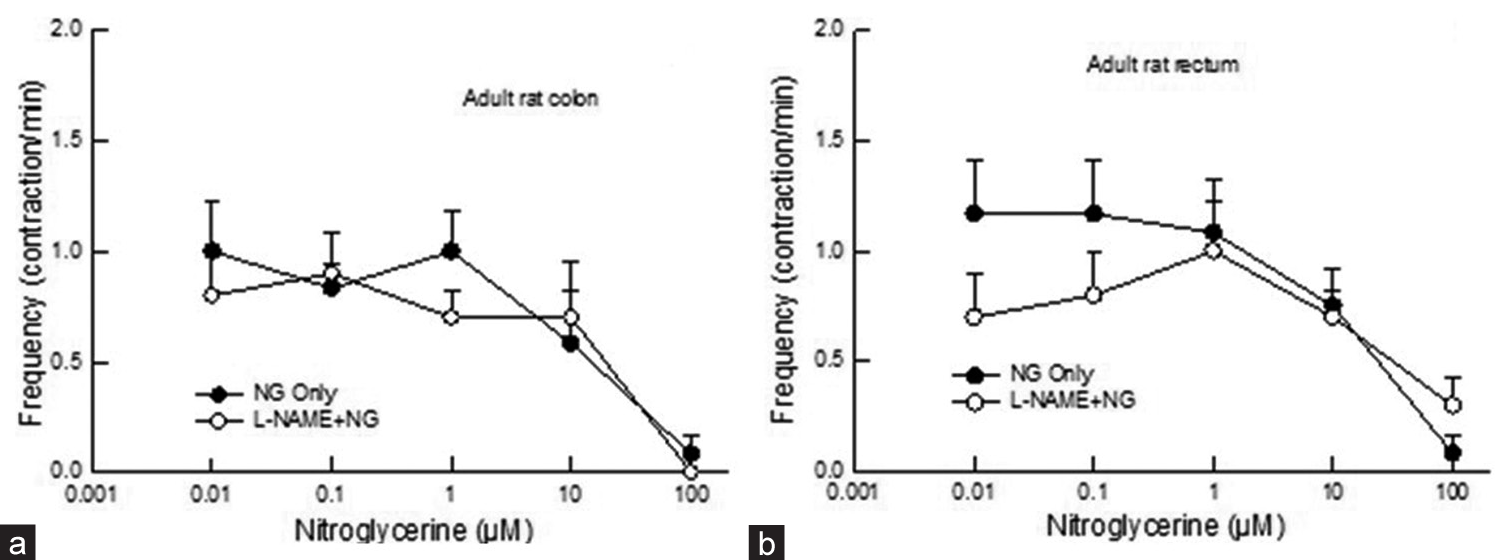
- Dose-response curve showing the effect of NG (0.01–100 mM) on contractile frequency in adult rat (a) colon (b) rectum before and after L-NAME application. Application of NG, did not show any difference in frequency in colon and rectum after application of L-NAME (P > 0.05, two-way Analysis of Variance, n = 5–6). Data points indicate mean ± standard error of the mean values. NG: Nitroglycerin, L-NAME: N--nitro-L-arginine methyl ester.
In neonate colon and rectum
In the neonate colon, apart from an increase in contractile frequency at low bath concentrations of NG, there was decrease in contractile frequency as the concentration of NG increased, and there was a more significant decrease in contractile frequency in the rectum than in the colon (P < 0.05, two-way ANOVA). However, no change in contractile frequency was found in the neonate rat colon and rectum to NG after L-NAME pre-treatment (P > 0.05, two-way ANOVA) [Figures 4a and b].
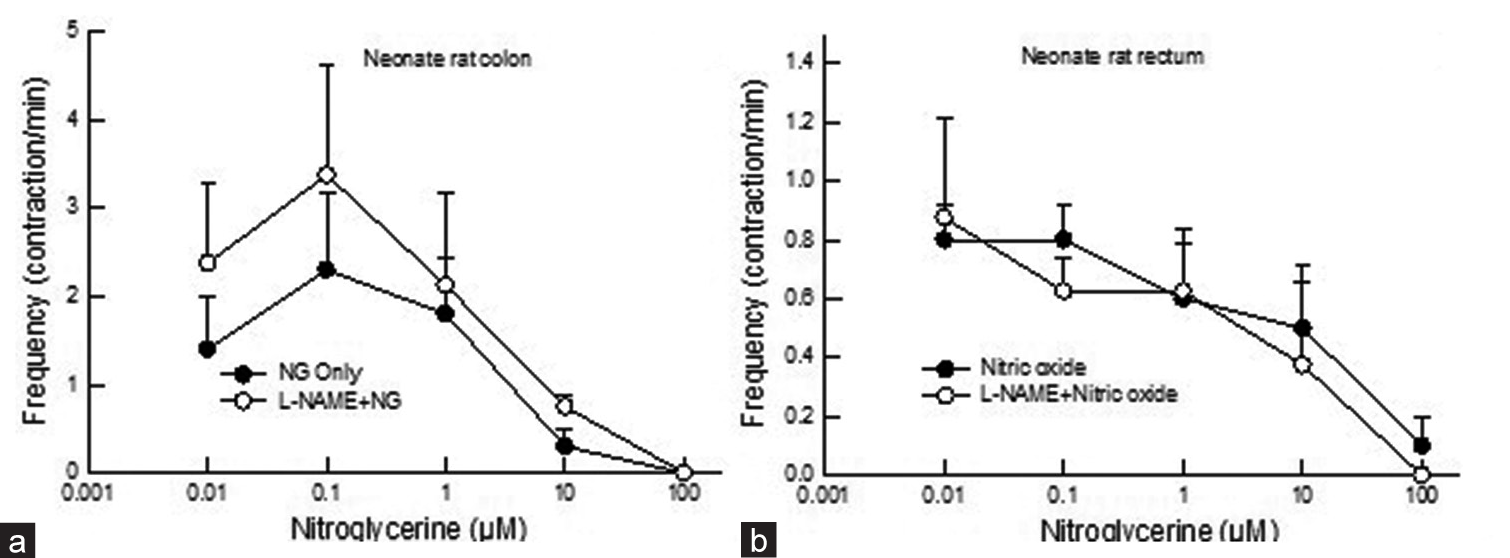
- Dose-response curve showing the effect of NG (0.01–100 mM) on contractile frequency in neonate rat (a) colon and (b) rectum before and after L-NAME application. The application of NG did not affect contractile frequency in neonate rat colons after the application of L-NAME (P > 0.05, two-way Analysis of Variance). Data points indicate the mean ± standard error of the mean values. NG: Nitroglycerin, L-NAME: N--nitro-L-arginine methyl ester.
DISCUSSION
The present study investigated the effect of NG, a NO donor, on colorectal contractility in rats. The study demonstrated a decrease in contractile tension in adult rat colon and rectum in a concentration-dependent manner with maximum relaxation at 100 mM of NG. Sensitivity to NO appeared more in the adult colon than in the rectum because the EC50 values in the adult colon and rectum were 4.2 mM and 15 mM, respectively. Relaxation of human colonic circular muscles by NG was reported earlier.[15] Other studies showed NO-mediated relaxation of gastrointestinal smooth muscle at various sites in several species.[2,16,17,19-21] There is a lack of data on the action of NG on rectal smooth muscle. This study demonstrates the action of NG on the rectum, showing similar dose-dependent activity in the colon and rectum, with greater sensitivity in the colon.
A NOS inhibitor was employed to understand further the presence of NO involvement in the modulation of colon and rectum contractility. When the colon and rectum of adult rats were pre-treated with L-NAME (a NOS inhibitor), it was observed that the relaxing effect of NG was obscured [Figures 1a and b]. L-NAME has also been shown to inhibit enzymes.[11,16] L-NAME per se also increased spontaneous contractile tension in the adult colon and rectum, indicating an endogenous NO activity in the tissue [Table 1]. Thus, our experiments confirmed observations on NO actions on colon and rectum contractility.[16,22]
The mechanism of NO action has also been seen to be mediated through the cyclic guanosine monophosphate (cGMP) pathway.[9,11]
Although there have been reports on the actions of NO in adults’ gut segments, its action on the neonate’s large gut remains to be investigated well. In the neonate colon and rectum, exposure to NG also caused relaxation, but unlike in adult rats, there was more relaxation in the rectum, that is, about 57% against 30% in the colon at 100 mM of NG. The EC50 value in the neonate’s colon was more than 100 mM, and in the rectum, EC50 was 35 mM, indicating that the neonate rectum is more sensitive to NO-induced relaxation than the colon. As a result, the reversal of sensitivity between the colon and rectum may have indicated a change in the NO-mediated mechanism of action from neonate to adulthood during the development process. As there is no study to date in neonate large gut to compare the contractile activity of the colon and rectum in any species, it is difficult to delineate the response for differential action on the colon and rectum. Nevertheless, our study revealed for the 1st time that nitrergic mechanisms might differ not only between the colon and rectum but also in neonates and adults.
In the present study, with neonate rat colon and rectum, L-NAME (100 mM) pre-incubation prevented the NG-induced relaxation [Figures 2a and b].
Furthermore, L-NAME per se increased spontaneous contractile tension in the neonate colon and rectum, suggesting the same basal NO activity as observed above in adult rats [Table 1].
Regarding the frequency of contractions, there has been a dose-dependent decrease in contractile frequency to NG, but there was no difference between the colon and rectum of adult rats. These observations suggest that NG had a similar effect on contractile frequency in adult rats’ colons and rectum. A decrease in contractile frequency was also observed in the small intestine of rabbits exposed to sodium nitroprusside, a NO donor.[8] Therefore, NO-induced changes in the frequency of spontaneous contraction are likely to be mediated by changing the functioning of interstitial cells of Cajal (ICC), as the frequency of contractions is determined by the pacemaker activity of ICC.[22-24] At present, it is difficult to speculate on the mechanism by which NO influences the activity of ICC. However, the influence of the NO-sensitive guanylyl cyclase signalling mechanism has been suggested.[22]
In neonates, similar decreases in the contractile frequency of the colon and rectum were also observed. However, in contrast to adults, the decline in frequency in the rectum was more profound. Again, this shows that the neonate rectum is more sensitive to NO, whether spontaneous contractile tension or frequency.
It is worth noting that L-NAME treatment had no effect on contractile frequency in adult and neonate rat colon and rectum [Figures 3a and b, 4a and b]. This suggests that L-NAME does not affect NG-induced frequency changes. These results align with earlier reports on small intestine contractile frequency in rabbits.[8] Further, at this point, it is difficult to determine the cause of the differential action of L-NAME on contractile frequency in the colon and rectum.
CONCLUSION
It may be concluded that nitrergic mechanisms are present from birth as NO-influenced contractile activity (both contractile tension and frequency) of large gut (colon and rectum) in adult and neonate rats. The basal NO release in the gut contributes to the inhibitory control of gut motility. Further, the intensity of control by NO may differ in the colon and rectum, with the colon being more sensitive to NO in adults. In contrast, the rectum is more susceptible to NO in neonates. The differences in NO sensitivity in adults and neonates also demonstrated the changes during the development process.
In the present study, the influence of the NO-sensitive guanylyl cyclase signalling mechanism was studied; however, the cGMP mechanism was not explored using cGMP inhibitors like methylene blue, and this is, therefore, a limitation in the study. Similarly, there is the possibility of the role of other relaxants, which was not studied in the present study.
Acknowledgement
UCG for financial assistance.
Ethical approval
Institutional review board (IRB) permission obtained for the study: Number: Dean/12-13/CAEC/32. Dated 30.6.2016.
Declaration of patient consent
The authors certify that they have obtained all appropriate consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
UGC.
References
- Enteric nervous system: Sensory transduction, neural circuits and gastrointestinal motility. Nat Rev Gastroenterol Hepatol. 2020;17:338-51.
- [CrossRef] [PubMed] [Google Scholar]
- Neurotransmitters: The critical modulators regulating gut-brain axis. J Cell Physiol. 2017;232:2359-72.
- [CrossRef] [PubMed] [Google Scholar]
- Akt phosphorylation of neuronal nitric oxide synthase regulates gastrointestinal motility in mouse ileum. Proc Natl Acad Sci U S A. 2019;116:17541-6.
- [CrossRef] [PubMed] [Google Scholar]
- Nitric oxide: From gastric motility to gastric dysmotility. Int J Mol Sci. 2021;22:9990.
- [CrossRef] [PubMed] [Google Scholar]
- Nitric oxide is essential for generating the minute rhythm contraction pattern in the small intestine, likely via ICC-DMP. Front Neurosci. 2020;14:592664.
- [CrossRef] [PubMed] [Google Scholar]
- Integrative control of gastrointestinal motility by nitric oxide. Curr Med Chem. 2016;23:2715-35.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of nitrergic system on colonic motility in a rat model of irritable bowel syndrome. Indian J Pharmacol. 2016;48:424-9.
- [CrossRef] [PubMed] [Google Scholar]
- The role of NO in the contractility of rabbit small intestine in vitro: Effect of K+ channels. J Physiol Pharmacol. 2005;56:407-19.
- [Google Scholar]
- Study on the cyclic GMP-dependency of relaxations to endogenous and exogenous nitric oxide in the mouse gastrointestinal tract. Br J Pharmacol. 2007;150:88-96.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanism of nitric oxide-induced contraction in the rat isolated small intestine. Br J Pharmacol. 1997;120:975-81.
- [CrossRef] [PubMed] [Google Scholar]
- The role of nitric oxide and L-type calcium channel blocker in the contractility of rabbit ileum in vitro. J Physiol Biochem. 2012;68:521-8.
- [CrossRef] [PubMed] [Google Scholar]
- Involvement of neuronal nitric oxide synthase (nNOS) in the regulation of migrating motor complex (MMC) in sheep. Vet J. 2012;192:352-8.
- [CrossRef] [PubMed] [Google Scholar]
- Role of nitric oxide-and vasoactive intestinal polypeptide-containing neurones in human gastric fundus strip relaxations. Br J Pharmacol. 2000;129:12-20.
- [CrossRef] [PubMed] [Google Scholar]
- Evidence that nitric oxide mechanisms regulate small intestinal motility in humans. Gut. 1999;44:72-6.
- [CrossRef] [PubMed] [Google Scholar]
- Involvement of nitric oxide in the inhibitory innervation of the human isolated colon. Gastroenterology. 1993;104:690-7.
- [CrossRef] [PubMed] [Google Scholar]
- Regional differences in the nitrergic innervation between the proximal and the distal colon in rats. Gastroenterology. 1998;115:1504-12.
- [CrossRef] [PubMed] [Google Scholar]
- Nitric oxide and vasoactive intestinal polypeptide mediate non-adrenergic, non-cholinergic inhibitory transmission to smooth muscle of the rat gastric fundus. Eur J Pharmacol. 1990;191:303-9.
- [CrossRef] [PubMed] [Google Scholar]
- Pouch colon associated with anorectal malformations fails to show spontaneous contractions but responds to acetylcholine and histamine in vitro. J Pediatr Surg. 2009;44:2156-62.
- [CrossRef] [PubMed] [Google Scholar]
- Release of nitric oxide upon stimulation of nonadrenergic noncholinergic nerves in the rat gastric fundus. J Pharmacol Exp Ther. 1991;256:441-7.
- [Google Scholar]
- Nitric oxide-mediated inhibitory response of rat proximal colon: Independence from changes in membrane potential. Br J Pharmacol. 1994;112:676-82.
- [CrossRef] [PubMed] [Google Scholar]
- Mediators of nonadrenergic, noncholinergic inhibition in the proximal, middle and distal regions of rat colon. Br J Pharmacol. 1993;108:348-55.
- [CrossRef] [PubMed] [Google Scholar]
- Nitrergic signalling via interstitial cells of Cajal regulates motor activity in murine colon. J Physiol. 2015;593:4589-601.
- [CrossRef] [PubMed] [Google Scholar]
- Colonic transit disorder mediated by downregulation of interstitial cells of cajal/anoctamin-1 in dextran sodium sulfate-induced colitis mice. J Neurogastroenterol Motil. 2019;25:316-31.
- [CrossRef] [PubMed] [Google Scholar]
- Nitrergic signaling via interstitial cells of Cajal and smooth muscle cells influences circular smooth muscle contractility in murine colon. Neurogastroenterol Motil. 2018;30:e13300.
- [CrossRef] [PubMed] [Google Scholar]






