Translate this page into:
Tocilizumab fails survival benefit in severe COVID-19 – A retrospective cohort study
*Corresponding author: Prasan Kumar Panda, Department of Medicine, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India. motherprasanna@rediffmail.com
-
Received: ,
Accepted: ,
How to cite this article: Arjun, Ranka R, Panda PK. Tocilizumab fails survival benefit in severe COVID-19 – A retrospective cohort study. Indian J Physiol Pharmacol 2022;66:55-61.
Abstract
Objectives:
Anti-interleukin-6 monoclonal antibody, tocilizumab, has produced mixed results in clinical trials for effectiveness against coronavirus disease 2019 (COVID-19). We conducted a retrospective cohort study to compare outcomes at 28 days of a cohort of patients with severe COVID-19 treated with tocilizumab and standard care, with those receiving standard care only.
Materials and Methods:
In this record-based retrospective cohort study, patients hospitalised with COVID-19 were classified into non-severe and severe disease as per institutional protocol. One cohort received tocilizumab with standard care and the second cohort received only standard care. Few patients also received high-dose steroids as ‘pulse’ steroids on initial clinical deterioration. Data were collected for the treatment given including oxygen interface, steroids, antimicrobials, duration of hospital stay in survivors, requirement of mechanical ventilation, and day of intubation from symptom onset. The primary outcome was to compare the all-cause mortality between the two groups. The effect of pulse steroid therapy on all-cause mortality was studied in the secondary outcome.
Results:
There was statistically significant mortality in the tocilizumab cohort as compared to standard care alone (HR 2.43, 95% CI 1.54–3.89). The need for mechanical ventilation was more in the tocilizumab cohort (85% vs. 18%, P < 0.001). Tocilizumab cohort had a delay in the day of intubation by a mean of 2.29 days from the day of symptom onset (P < 0.05). Pulse steroid administration showed increased all-cause mortality (HR 1.94, 95% CI 1.18–3.20) and risk of mechanical ventilation.
Conclusion:
Tocilizumab cohort showed higher mortality and need for mechanical ventilation in our study which contrasts the result of a few previous trials. Our study warrants the need for future clinical trials on this subject to ensure better treatment strategies in upcoming COVID-19 waves.
Keywords
Coronavirus disease
Experimental therapy
Interleukin-6 inhibitor
Pulse steroid
INTRODUCTION
Coronavirus disease 2019 (COVID-19) emerged in Wuhan, China, in December 2019 and rapidly led to a public health emergency.[1] The current treatment guidelines by the Infectious Disease Society of America recommend only the use of corticosteroids in severe COVID-19 pneumonia.[2] Novel therapies targeting different pathways of viral replication and suppression of immune response are under development to reduce the global health impact of this worldwide pandemic.[3]
COVID-19 virus leads to pneumonia, acute respiratory distress syndrome and finally hypoxemic failure.[4] The pathophysiology of the disease highlights development of severe immune response resembling cytokine release syndrome (CRS), leading to increased production of interleukin-6 (IL-6), ferritin, C-reactive protein and D-dimer among other inflammatory markers.[5,6] Increased levels of IL-6 have been associated with increased viremia, enhanced RNA shedding, and overall increased disease progression and mortality.[6]
Tocilizumab is a humanised monoclonal antibody selectively targeting IL-6 receptors, which recently became an option for the treatment of CRS.[7,8] Few clinical trials have studied the effect of tocilizumab on COVID-19 on mortality and clinical progression with mixed results. The RECOVERY trial showed mortality benefit among non-ventilated COVID-19 patients but not in those with mechanical ventilation.[8] A meta-analysis by Shao et al. shows no statistical difference in mortality for patients receiving tocilizumab versus those with standard care.[9] Other clinical trials and some observational studies also provide conflicting data regarding the mortality benefit, especially in patients with severe disease.[10] The results have also varied among different ethnicities and critical care setups.[11]
We conducted a retrospective cohort study to compare outcomes at 28 days of a cohort of patients with severe COVID-19 treated with tocilizumab and standard care, with those concomitantly hospitalised and receiving standard care only.
MATERIALS AND METHODS
Study design and setting
It is a record-based retrospective cohort study in a setting of a tertiary care teaching government hospital (1000 bedded) in North India. Patients hospitalised with COVID-19 between June 2020 and March 2021 were eligible for inclusion. The Institutional Ethics Committee, AIIMS, Rishikesh, approved the study (No. 402/IEC/IM/NF/2020). The data collection was done through the patient’s medical e-records on the National Informatics Centre’s e-hospital portal being currently used by the hospital.
Study population and patient selection
All adult (>18 years) hospitalised patients diagnosed with COVID-19 who got admitted within the study period were taken as the study population. COVID-19 positivity was defined as having confirmed reverse transcriptase polymerase chain reaction positivity for SARS-CoV-2 on a nasopharyngeal swab or clinical features and chest radiological findings highly suggestive of COVID-19 infection with no other explainable diagnosis. The patients were classified into non-severe and severe disease as per institutional protocol corresponding to the WHO guideline. The patients who were included in other ongoing trials and those who had refused consent for the use of clinical data for research purposes at the time of admission to the hospital were excluded from the study. The two cohorts for the study included one cohort receiving tocilizumab with standard care and the second comparison cohort receiving only standard care as per institutional protocol. Standard care comprised paracetamol, oxygen, anti-allergy and antibiotics as indicated, anticoagulants, steroids, and other antivirals – remdesivir for selective patients. A few patients also received high-dose steroids as ‘pulse’ steroids (dexamethasone 40 mg OD or methylprednisolone 250–500 mg OD for 3–5 days and then tapered) when high CT severity scores > 24/40 or clinical deterioration after normal dose steroid. A total of 38 patients were included in the tocilizumab cohort and 130 patients were included in the non-tocilizumab cohort.
Study variables
We collected all the baseline characteristics of relevance including demographics, symptoms, duration of illness, preexisting comorbidities, and medications. All the patients were cohorted into non-severe and severe categories. Non-severe cases included patients of mild-moderate severity. Severe cases required oxygen persistently (>24 h) at the time of hospitalisation. Oxygen delivery was through a nasal cannula, Hudson face masks, non-rebreather face masks, high-flow nasal cannula, non-invasive mechanical ventilation, and invasive mechanical ventilation.
From the hospital course, data were collected for the treatment given including oxygen interface, steroids, antimicrobials, and experimental therapies. Duration of hospital stay (in days) was calculated in survivors along with clinical recovery from symptom onset. Data were also collected for the requirement of mechanical ventilation and day of intubation from symptom onset. Laboratory values studied were D-dimer (mg/l), IL-6 (pg/ml), ferritin (ng/ml), hs-CRP and CT severity index score (out of 40).
The inclusion criteria for tocilizumab therapy as per institutional protocol were patients deteriorating after 48 h of steroid intake with IL-6 levels >40 (or hs-CRP >75). Patients with any other active infection; chronic steroid use; <500 neutrophils or <50 × 109 platelets; severe haematological, kidney or liver function impairment, or any clinical condition that could predispose to bowel perforation were excluded from the administration of the drug. The drug was administered at a dose of 8 mg/kg (max. dose 800 mg per dose) as a single dose or two doses in some circumstances when the clinical response was not achieved 1 day after administration. All patients were screened for latent tuberculosis infection through the Mantoux test or before the drug administration.
Outcomes
The primary outcome studied included all-cause mortality within 28 days of hospitalisation or till discharge (whichever was longer). Time to clinical recovery (in days) was studied in survivors. The non-severe category patients were analysed for the need for mechanical ventilation (in days) from the day of symptom onset. Secondary outcomes included the association of pulse steroid administration with all-cause mortality and the role of biochemical markers such as IL-6, ferritin, and D-dimer as predictors of mortality.
Statistical analysis
We compared the baseline characteristics of the patients treated with standard care with tocilizumab and those treated with standard care alone, including age, sex, existing comorbidities, pulse steroid use, biochemical and radiological parameters. Categorical variables were expressed as fractions (%) and compared using the Chi-squared test across the cohorts. Continuous variables were expressed as mean ± standard deviation and analysed using independent t-test between two cohorts and analysis of variance test for > two cohorts. Relationships were assessed using Pearson or Spearman tests depending on distribution type. Multivariate regressions (logistic for categorical and linear for continuous dependent variables) were used to determine the significance of predictor variables.
We did a survival analysis, following up patients from the date of symptom onset to death. We compared the time to death by treatment cohorts using unweighted Kaplan–Meier curves and univariate and multivariate Cox regression analysis. The effect of treatment was studied using an unadjusted and adjusted hazard ratio (HR) with a 95% CI. Finally, a forest plot was constructed for multiple variables as the role of predictors using HR and 95% confidence intervals.
We considered a two-sided P-value test of <0.05 to be statistically significant. The data were entered into the Microsoft Excel spreadsheet and analysis was done using IBM Statistical Package for the Social Sciences version 26.0 (Chicago, US).
RESULTS
Among patients screened in record sections during the study period (n = 325), 150 patients were excluded from the study (135 were already enrolled in other studies and 15 had missing forms). Out of the remaining 175, 42 received at least a single dose of tocilizumab, and 133 did not receive any dose. Four among the tocilizumab cohort and three among the non-tocilizumab cohort had the majority of variables missing and hence were excluded from the study. Finally, 38 patients in the tocilizumab cohort and 130 patients in the non-tocilizumab cohort were included for per-protocol analysis [Figure 1].

- The study flow.
Out of a total of 168 patients, 126 (75%) were male. The mean age was 54.90 ± 15.09 (SD) years and 41% had either hypertension or diabetes mellitus. A total of 34 patients also received pulse steroids, but the number was more in the tocilizumab cohort (47.4%) as compared to the nontocilizumab cohort (12.3%). A total of 20.23% of patients belonged to the non-severe category which required < 1-day oxygen delivery. The majority of the patient’s required high-flow oxygen or mechanical ventilation at the time of admission but the proportion was more in the tocilizumab cohort (92%) as compared to the non-tocilizumab cohort (76.2%) [Table 1]. Overall, more severe patients were included in the tocilizumab cohort, which can be explained by the hospital indications for the use of tocilizumab in very sick patients with high inflammatory markers.
| Tocilizumab with standard care (n=38) | Standard care (n=130) | Overall | P-value | |
|---|---|---|---|---|
| Age (Mean±SD) | 56.62±14.73 | 54.20±15.20 | 54.90±15.09 | 0.349 |
| Sex (%) | ||||
| Male | 28 (73.7%) | 98 (75.4%) | 126 (75%) | 0.833 |
| Female | 10 (26.3%) | 32 (24.6%) | 42(25%) | |
| Comorbidities (%) | ||||
| Hypertension | 17 (44.7%) | 52 (40%) | 69 (41%) | 0.520 |
| Diabetes | 13 (34.2%) | 56 (43.1%) | 69 (41%) | 0.331 |
| Heart disease | 5 (13.2%) | 25 (19.2%) | 30 (17.87%) | 0.427 |
| Pulse steroid use (%) | 18 (47.4%) | 16 (12.3%) | 34 (20.23%) | <0.001 |
| Key time variables – (Mean±SD) | ||||
| T to recovery (in survivors)* | 26.75±11.92 | 20.13±8.88 | 20.41±9.05 | 0.154 |
| Day of intubation (from symptom onset) | 17.79±6.53 | 15.5±6.07 | 17.04±6.40 | 0.245 |
| Clinical severity (%) | ||||
| Non-severe | 03 (7.9%) | 31 (23.8%) | 34 (20.23%) | <0.05 |
| Severe | 35 (92%) | 99 (76.2%) | 134 (79.76%) | |
| Laboratory values | ||||
| IL-6 | 265±413 | 105.94±262 | 161±329 | 0.041 |
| D-dimer | 2.39±3.23 | 2.11±3.07 | 2.21±3.11 | 0.685 |
| Ferritin | 1114±747 | 1027.43±938 | 1056±871 | 0.756 |
| Survivors | 4 (10%) | 90 (69.23%) | 94 (47.47%) | <0.01 |
| Case fatality rate (%) | 89.47% | 30.76% | 44% |
Data are represented as mean±SD for continuous variables and as total (proportion %) for categorical variables. P-values mentioned are for two-tailed independent samples t-test. * T=Time to recovery in survivors in days
In a secondary analysis, the tocilizumab cohort had a delay in the day of intubation by a mean of 1.21 days from the time of admission and 2.29 days from the day of symptom onset (P < 0.05); pulse steroid administration showed increased all-cause mortality and risk of mechanical ventilation compared to the cohort without the use of pulse steroids (P < 0.05) and mean values of D-dimer, ferritin, and IL-6 were more in the mortality cohort as compared to discharged alive cohort but statistically significant for ferritin only [Table 2].
| Secondary outcomes | Tocilizumab plus standard care (n=38) | Standard care (n=130) | t-statistic | P-value |
|---|---|---|---|---|
| Day of intubation from the time of admission | 10.21±6.39 | 9.00±4.47 | 0.68 | 0.500 |
| Day of intubation from symptom onset | 17.79±6.51 | 15.50±6.07 | 1.17 | 0.245 |
| Pulse steroid used | Pulse steroid not used | Chi-squared statistic | ||
| All-cause mortality | 24 (70%) | 50 (59%) | 12.18 | <0.001 |
| Need for mechanical ventilation | 10 (55%) | 24 (26%) | 11.60 | 0.003 |
| Death | Discharged alive | t- statistic | ||
| D-dimer (mg/L) | 2.58±2.79 (n=53) | 1.71±3.47 (n=40) | 1.326 | 0.188 |
| Ferritin (ng/mL) | 1391.84±1013.53 (n=25) | 637.15±360.04 (n=20) | 3.168 | 0.03 |
Data are n (%) or mean±SD and P-values refer to differences between overall tocilizumab and standard of care and were calculated using Chi-square test. Pearson’s Chi-squared coefficient has been mentioned. Independent samples t-test has been used for t statistic. P-value is two tailed with equal variances assumed
A Kaplan–Meier curve was constructed with a period of duration from the day of symptom onset to a hospital stay with events as mortality and estimated the cumulative probability of death and compared in-between tocilizumab versus non-tocilizumab cohorts showed the increased cumulative mortality former [Figure 2a]. Similarly, increased cumulative mortality in the pulse steroid cohort was there when compared to the non-pulse steroid cohort [Figure 2b].

- Kaplan–Meier estimators of survival rate between tocilizumab and non- tocilizumab cohorts (a) and pulse steroid therapy and non-pulse steroid cohorts (b).
The HR in the forest plot was adjusted for multiple covariates [Figure 3]. Patients treated with tocilizumab had a higher ratio (HR 2.43, 95% CI 1.54–3.89) while that for pulse steroid therapy too had a statistically significant trend toward increased mortality (HR 1.94 95% CI 1.18–3.12).
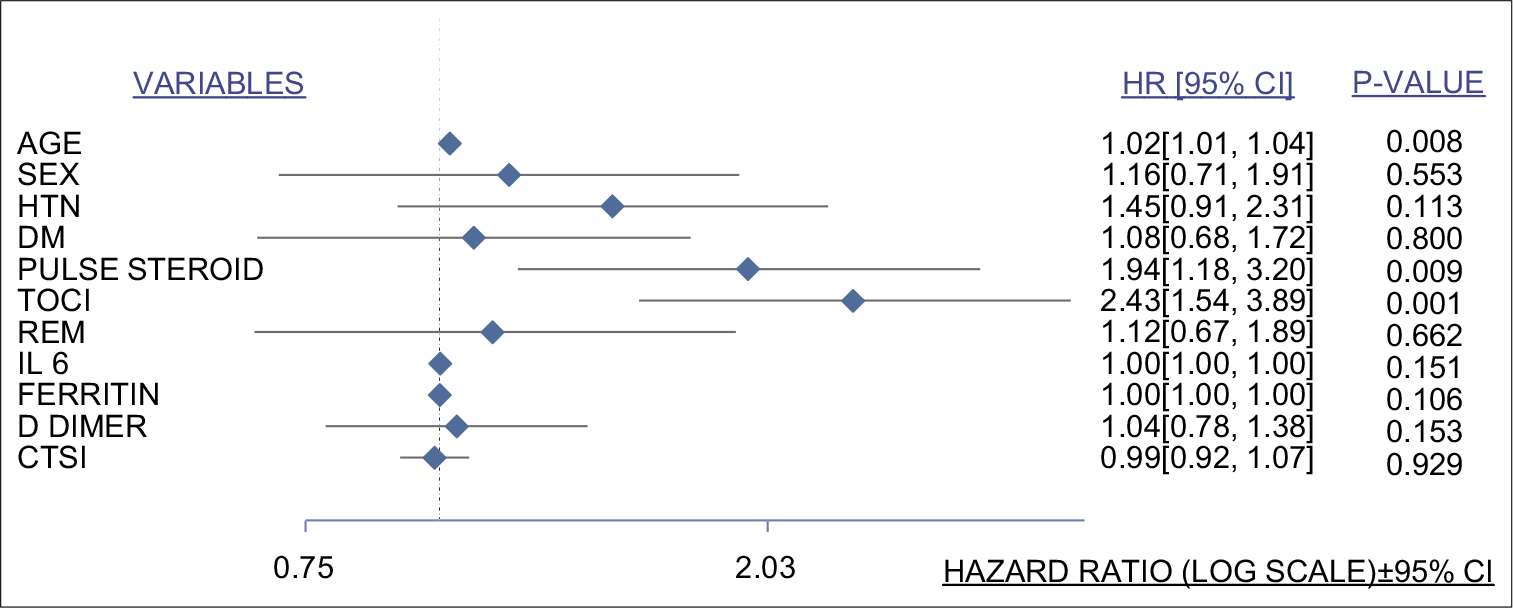
- Forest plot for multivariate analysis. Variables studied listed on the left side and hazard ratio with 95% confidence interval along with their corresponding P-values on the right side calculated from linear and logistic regression models. HTN: Hypertension, DM: Diabetes mellitus, TOCI: Tocilizumab, REM: Remdesivir, IL-6: Interleukin-6, CTSI: Computed tomography severity index for COVID-19.
DISCUSSION
The retrospective study describes the real-life effectiveness of tocilizumab in severe COVID-19 patients in an Indian tertiary care hospital compared to patients receiving standard care. At 28 days, the cohort receiving tocilizumab endured higher mortality as compared to the standard care cohort (88% vs. 39%, P < 0.001) in the severe category. The tocilizumab cohort also showed a higher risk of progression to mechanical ventilation (85% vs. 18%, P < 0.001).
Since the mid-2020s, there have been many RCTs comparing tocilizumab as a treatment for COVID-19, the combined result of which provides a mortality benefit (RR 0.91, CI 0.83–0.99).[8] The RECOVERY trial showed an overall mortality benefit (HR 0.86, CI 0.71–0.96), but this was not consistent with those requiring mechanical ventilation at randomisation (HR 0.94, CI 0.73–1.19).[12] A similar retrospective analysis by Quartuccio et al. concluded both increased mortality (HR 14.65, CI 0.81–265.46) and risk of invasive ventilation (HR 3.87, CI 1.76–8.52) in the tocilizumab cohort.[13]
COVID-19 disease may be associated with a dysregulated immune response, leading to acute respiratory distress syndrome and multiorgan failure.[14] A strong correlation between serum IL-6 levels and ongoing hypoxemic respiratory failure has been hypothesised. Increased viral RNA shedding has also been found to be related to increased IL-6 levels. IL-6 promotes TH17 activation, leading to neutrophil migration, and acts as a pyrogenic for a thermostatic response.[15] It is also associated with increased IL-1b and TNF activation, leading to increased tissue permeability and oedema. IL-6 levels may be sub-maximally elevated in COVID-19 and it cannot be determined whether they merely represent a marker for severity or can be targeted for therapeutic interventions.[16] Thus, the IL-6 blockade may act as a double-edged sword.
In an RCT by Maryam et al., intravenous methylprednisolone pulse was associated with a survival benefit (HR 0.293 95% CI 0.154–0.556, P < 0.001)) in non-mechanically ventilated hospitalised COVID-19 patients without comorbidities.[17] In the present study, pulse steroids use with dexamethasone was associated with higher mortality (HR 1.94, 95% CI 1.18–3.20, P < 0.05). These patients had severe disease and several patients suffered from comorbidities including diabetes, hypertension, and chronic cardiac disease which would have resulted in an adverse outcome in this group, as depicted in [Figure 2b]. Serum ferritin values were observed to be higher in the tocilizumab group, the possible explanation for which can be the higher number of severe category patients in that group as compared to the standard treatment group and ferritin being an acute-phase reactant.
As depicted in [Figure 2a], tocilizumab administration was associated with higher mortality as compared to standard care. Higher baseline severity in the tocilizumab group is one possible explanation. Increased predisposition to infections in this subgroup resulting from blockade of the inflammatory cascade may have contributed to sepsis and increased mortality as depicted in other studies.[18] [Figure 3] depicts the relationship between the increased adverse effects of tocilizumab and pulse steroids on all-cause mortality in this study. As highlighted by the previous studies, IL-6 and ferritin levels were higher in those with severe disease and progressing to mortality or invasive ventilation.
The present study has certain limitations. First, the retrospective design fails to rule out unmeasured confounders. Several patients have concomitant steroid administration with varying doses and duration that make interpretation of results difficult. Second, the tocilizumab cohort includes a higher number of patients with clinically severe disease and more patients requiring mechanical ventilation and with pulse steroid administration. Finally, the adverse events in both cohorts have not been measured which might have brought some clarity to these conflicting results.
CONCLUSION
It is a large study including patients from real-life hospital settings in midst of a pandemic in a developing nation. It highlights the challenges that newer therapies face and the developments needed for us to tackle upcoming waves of COVID-19. Tocilizumab emerged as an attractive weapon against the virus but the guidelines for its use including the appropriate dosing and timing of administration may need further revision. The results of this study should guide us to conduct more RCTs, especially in developing nations, and involve different ethnic cohorts to ascertain the role of novel drugs in the treatment of COVID-19.
Acknowledgment
Thanks to the COVID-19 management team for patient care and helping data collection.
Authors’ contributors
Arjun and RR contributed to the data collection, their analysis and were involved in manuscript writing. PKP gave the concept, interpreted analysis, critically reviewed the draft, and approved it for publication along with all authors.
Data sharing
It will be made available to others as required on requesting the corresponding author.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- COVID-19 Public Health Emergency of International Concern (PHEIC) Global Research and Innovation Forum. Available from: https://www.who.int/publications/m/item/covid-19-public-health-emergency-of-international-concern-(pheic)-global-research-and-innovation-forum [Last accessed on 2021 Jul 20]
- [Google Scholar]
- IDSA Guidelines on the Treatment and Management of Patients with COVID-19. Available from: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management [Last accessed on 2021 Jul 20]
- [Google Scholar]
- Recent developments on therapeutic and diagnostic approaches for COVID-19. AAPS J. 2021;23:14.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19: Virology, biology and novel laboratory diagnosis. J Gene Med. 2021;23:e3303.
- [CrossRef] [PubMed] [Google Scholar]
- The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection. J Allergy Clin Immunol. 2020;146:518-34.e1.
- [CrossRef] [PubMed] [Google Scholar]
- Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis. Clin Chem Lab Med. 2020;58:1021-8.
- [CrossRef] [PubMed] [Google Scholar]
- FDA approval summary: Tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 2018;23:943.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of tocilizumab in hospitalized COVID-19 patients: A systematic review and meta-analysis. J Infect. 2022;84:418-67.
- [CrossRef] [PubMed] [Google Scholar]
- Tocilizumab for severe COVID-19: A systematic review and meta-analysis. Int J Antimicrob Agents. 2020;56:106103.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383:2333-44.
- [CrossRef] [PubMed] [Google Scholar]
- Racial and gender-based differences in COVID-19. Front Public Health. 2020;8:418.
- [CrossRef] [PubMed] [Google Scholar]
- Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637-45.
- [CrossRef] [Google Scholar]
- Profiling COVID-19 pneumonia progressing into the cytokine storm syndrome: Results from a single Italian Centre study on tocilizumab versus standard of care. J Clin Virol. 2020;129:104444.
- [CrossRef] [PubMed] [Google Scholar]
- Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol. 2020;108:17-41.
- [CrossRef] [PubMed] [Google Scholar]
- Is IL-6 a key cytokine target for therapy in COVID-19? Nat Rev Immunol. 2021;21:337-9.
- [CrossRef] [PubMed] [Google Scholar]
- IL-6: Relevance for immunopathology of SARS-CoV-2. Cytokine Growth Factor Rev. 2020;53:13-24.
- [CrossRef] [PubMed] [Google Scholar]
- Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: Results from a randomised controlled clinical trial. Eur Respir J. 2020;56:2002808.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and secondary infection risk of tocilizumab, sarilumab and anakinra in COVID-19 patients: A systematic review and meta-analysis. Rev Med Virol 2021:e2295.
- [CrossRef] [PubMed] [Google Scholar]






