Translate this page into:
Comparison of autonomic function tests and high-sensitivity C-reactivity protein in overweight patients of polycystic ovarian syndrome and overweight controls
*Corresponding author: Dr. Supriya Gupta, Director Professor, Department of Physiology, Vardhman Mahavir Medical College and Safdarjung Hospital, Ansari Nagar, New Delhi. guptasupriya59@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Pattnaik S, Gupta S, Saxena U, Matlani M, Kapoor R. Comparison of autonomic function tests and high-sensitivity C-reactivity protein in overweight patients of polycystic ovarian syndrome and overweight controls. Indian J Physiol Pharmacol 2020;64(4):303-8.
Abstract
Objectives:
Both polycystic ovarian syndrome (PCOS) and high body mass index (BMI) are associated with autonomic dysfunction. Most of the patients of PCOS have high BMI. Hence, BMI is likely to be a factor contributing to the autonomic dysfunction in PCOS. High-sensitivity C-reactive protein (hs-CRP) is a marker of inflammation and a predictor of future cardiovascular risk. PCOS patients have low-grade chronic inflammatory state. Coexistence of PCOS and obesity causes more increase in CRP, thereby further increasing the risk of cardiovascular morbidity. We have performed autonomic function tests and estimated hs-CRP in overweight patients of PCOS and compared our findings with the control group consisting of overweight normal subjects. Our aim is to find out whether the cardioautonomic and inflammatory changes seen in PCOS are due to the presence of increased weight or do the presence of increased weight add to the severity of these changes seen in PCOS.
Materials and Methods:
Cases consisted of 44 overweight patients of PCOS and controls included 44 healthy overweight subjects, all in the age group of 18–45 years. Autonomic function test consisted of three sympathetic and three parasympathetic reactivity tests. The following tests were done for parasympathetic reactivity: (a) Deep breathing test – expiration:inspiration ratio (E:I) was calculated. (b) Valsalva maneuver-Valsalva ratio was calculated. (c) Heart rate changes from lying to standing (LST) - 30:15 ratio was calculated. For assessing sympathetic reactivity, the following tests were used: (a) Isometric handgrip test – the difference between resting diastolic blood pressure (DBP) reading and the reading before release of hand grip is noted. (b) Cold pressor test (CPT) – The rise in DBP over the baseline DBP was noted. (c) Systolic BP (SBP) change in LST – the maximum fall in SBP was recorded. Measurement of serum hs-CRP was done using enzyme-linked immunosorbent assay.
Results:
We found that in the overweight PCOS group, there was a significant decrease in both sympathetic and parasympathetic reactivity than the overweight control group (p=0.000 for Valsalva ratio, 0.027 for 30:15 ratio, and 0.0005 for CPT). The difference between hs-CRP in controls and PCOS was also significant (P = 0.039).
Conclusion:
In our study, we concluded that the pathological changes due to PCOS could be attributed directly to the extent of inflammation measured by hs-CRP levels. These changes were not directly related to BMI as proven by comparing with controls (overweight non-PCOS subjects).
Keywords
Autonomic dysfunction
Polycystic ovarian syndrome
Sympathetic reactivity
Parasympathetic reactivity
High-sensitivity C-reactive protein
INTRODUCTION
Autonomic dysfunction is an early marker of cardiovascular morbidity and mortality. Studies have reported the presence of cardiac autonomic dysfunction in patients of polycystic ovarian syndrome (PCOS), which is one of the most common endocrine disorders in women of the reproductive age group.[1-6] The study also reveals the presence of autonomic dysfunction with increasing body mass index (BMI).[7] As women with PCOS generally have greater BMI, they pose a greater risk of autonomic dysfunction. Hence, whether the autonomic dysfunction in PCOS is due to the increased BMI or due to the underlying disease pathology is debatable.
C-reactive protein (CRP) is one of several acute-phase reactants and a major circulating biomarker of inflammation. CRP has been proven to be a strong independent predictor of cardiovascular events in healthy asymptomatic as well as symptomatic PCOS patients.[8-10] Although the presence of obesity is closely associated with elevations in CRP, studies have shown that CRP is elevated in PCOS independent of obesity.[11,12] Coexistence of PCOS and obesity causes more increase in CRP, thereby further increasing the risk of cardiovascular morbidity.[13]
Our aim is to find out whether the cardioautonomic and inflammatory changes seen in PCOS are due to the presence of increased weight or do the presence of increased weight add to the severity of these changes seen in PCOS. Although there are some studies comparing the sympathetic and parasympathetic reactivity tests in PCOS in the obese and lean population, very limited study has been done in overweight category that too in the Indian population. There are not many studies on correlation of high-sensitivity CRP (hs-CRP) with the autonomic reactivity.
MATERIALS AND METHODS
After the Institutional Ethics Committee approved our study protocol, the subjects were recruited in 2018–2019 from our hospital’s outpatient’s department. The test procedures were explained to the subjects after which they gave voluntary written consent.
Study design
Sample size was calculated by considering the standard deviations of means of values of heart rate (HR) variability of cases and control group as 27.47 and 16.22, respectively, as per the study of Hashim et al.[5] Taking the confidence interval as 95%, power as 80%, margin of error as ±20%, and non-response error as 10%, the minimum sample size comes out to be 44 in each group using the formula:
Zα and Zβ are the values of α-error and β-error, respectively, and S1 and S2 are the values of standard deviation of means of HR rate variability of cases as well as controls and d is the power of the study.
It was a cross-sectional study conducted in 44 overweight patients (BMI ≥ 23) of PCOS and 44 age-matched healthy females who were overweight (BMI ≥ 23), with regular menstrual cycle and without clinical hyperandrogenism served as controls. All of them were in the age group of 18– 45 years. Pregnant and lactating women and the women on any kind of medication, including hormonal contraceptives, were excluded from the study.
PCOS diagnosis was based on the revised Rotterdam criteria, according to which any two of the following three criteria should be fulfilled for a case to be PCOS:
Oligoovulation or anovulation
Hyperandrogenism (either clinical or biochemical or both)
Polycystic ovaries and exclusion of other etiologies.[14]
Procedure
All participants were inquired about the history of illness and underwent anthropometric and general physical examinations. The test was conducted in a semi-dark and silent room. All recordings were obtained between 9 a.m. and 1 p.m. after an overnight fasting.
Patients and healthy controls were relaxed and remained in recumbent position during the recordings. The blood pressure (BP), HR, and electrocardiogram (ECG) were recorded for each participant after 10–15 min of rest. BP and HR were recorded using digital sphygmomanometer (OMRON) and ECG was recorded in BIOPAC MP150 using AcqKnowledge 4.3 software. The calculations were done manually [Annexure 1].
The following autonomic function tests were carried out to assess the cardiovascular reactivity.[15]
Parasympathetic reactivity tests:
Deep breathing test – Expiration:inspiration ratio (E:I ratio) During the test, the subjects were seated comfortably asked to breathe deeply and evenly at 6 breaths/ min (5 s in and 5 s out). The ECG was continuously recorded. The expiration:inspiration ratio (E:I) was calculated by dividing the average of maximum RR intervals during expiration by the average of minimum RR intervals during inspiration.[15]
Valsalva maneuver-Valsalva ratio (VR) They were instructed to blow into a mouth piece attached to a sphygmomanometer such that expiratory pressure was maintained at 40 mmHg and to hold the pressure for 15 s. At the end of 15 s, the pressure was released. ECG from lead II was recorded for 1 min to get the baseline values and continuously recorded during the maneuver and 45 s following release of respiratory strain.[15] 1VR = Longest R-R interval after maneuver (Phase IV)/Shortest R-R interval during test (Phase II).
HR changes from supine to standing (30:15 ratio) The subjects were asked to lie quietly on a couch under resting conditions and then to stand up unaided as quickly as possible and remain standing quietly for 1 min. The 30:15 ratio was calculated as the longest R-R interval around the 30th beat after standing divided by the shortest R-R interval around the 15th beat.[15]
-
Sympathetic reactivity tests
Diastolic BP (DBP) change during isometric handgrip test (HGT) Baseline BP was recorded in the subjects using digital sphygmomanometer. Then, they performed maximum voluntary contraction (MVC) by gripping the hand grip dynamometer with dominant hand, as hard as possible for few seconds and maximum force exerted was noted down. The subjects were asked to use their dominant hand to press the dynamometer at 30% of their MVC for 4 min. The difference between resting DBP reading and the reading before release of hand grip is taken as ΔDBP in HGT.[15]
DBP changes during cold pressor test (CPT) For CPT, baseline BP and HR recorded in all the patients in sitting position. The patients were then instructed to immerse the non-dominant hand till the level of wrist in cold water for 1 min which was maintained at a temperature of 10°C. The subject was instructed to inform and take his hand out of the water bath at any point of time in case of sensation of local or generalized discomfort or pain. The BP was measured at the end of 1 min. The rise in DBP over the baseline DBP was noted.[15]
Systolic BP (SBP) changes from lying to standing The test was performed by measuring the BP of all the subjects with a digital sphygmomanometer while lying down quietly for 15 min and again on standing up at 1st, 2nd, 2.5th, 3rd, and 5th min. The fall in SBP was recorded.[15]
Biochemical assay
All blood samples were obtained in the morning after overnight fasting. Three milliliters venous blood sample was collected in gel lined vial for serum separation for the estimation of high-sensitivity CRP. The blood was centrifuged for separating the serum and plasma, which were then stored at −20°C till the time of biochemical assays.
The measurement of serum hs-CRP levels in the subjects was performed using enzyme-linked immunosorbent assay (ELISA) of Calbiotech, Inc. manufacturer. The hsCRP ELISA is a typical two-step capture or sandwich-type assay. The procedure as per the kit literature was followed. Two highly specific monoclonal antibodies were involved: A monoclonal antibody specific for CRP was immobilized onto the microwell plate and another monoclonal antibody specific for a different region of CRP is conjugated to horseradish peroxidase (HRP). CRP from the sample and standards is allowed to bind to the plate, washed, and then incubated with the HRP conjugate. The substrate for enzyme is added after a second washing step. A stopping solution is added to terminate the enzymatic reaction. The absorbance is measured on a microtiter plate reader. The enzymatic reaction produces a color which is directly proportional to the CRP concentration in the sample.
Statistical analyses
Statistical analyses were performed using SPSS version 21 (SPSS, Inc., Chicago, IL). Data are expressed as mean ± standard deviation for continuous variables and as counts and proportions for categorical variables. Normally distributed parameters were compared using unpaired t-test, and Mann–Whitney U-test was used for parameters with non-normal distribution. P < 0.05 was considered to be statistically significant and P < 0.001 very significant.
RESULTS
The mean resting SBP in PCOS was 111.09 ± 7.50 mmHg and in the control group was 102.91 ± 8.54 mmHg. This showed a significant difference (P = 0.00001) among the two groups. The resting mean DBP in PCOS and control groups was 73.48 ± 7.15 mmHg and 73.95 ± 6.05 mmHg, respectively. The DBP showed no significant difference (P value = 0.736). The mean HR in PCOS and control group was 78.09 ± 6.30 beats per minute and 78.72 ± 7.64 beats per minute, respectively, with no significant difference (P = 0.677). The result of the sympathetic and parasympathetic reactivity tests is summarized in [Table 1]. They are classified according to the AIIMS AFT lab criteria [Table 2] according to which the three sympathetic and three parasympathetic reactivity tests are analyzed separately and are classified as follows:
| Test | Normal (score 0) | Borderline (score 1) | Abnormal (score 2) | Controls | PCOS | Pvalue |
|---|---|---|---|---|---|---|
| E:I ratio (DBT) | ≥1.21 | 1.11–1.20 | ≤1.10 | 1.51±0.256 | 1.42±0.168 | 0.057 |
| Valsalva ratio | ≥1.21 | 1.11–1.20 | ≤1.10 | 1.80±0.41 | 1.38±0.28 | 0.0000** |
| 30:15 ratio (LST) | ≥1.04 | 1.01–1.03 | ≤1.00 | 1.35±0.26 | 1.22±0.25 | 0.027* |
| HGT | ≥16 mmHg | 11–15 mmHg | ≤10 mmHg | 19.14±10.23 | 16.34±8.96 | 0.176 |
| CPT | ≥16 mmHg | 11–15 mmHg | ≤10 mmHg | 16.75±7.04 | 10.11±9.12 | 0.0005** |
| ΔSBP (LST) | ≤10 mmHg | 11–20 mmHg | >20 mmHg | 9.02±3.91 | 10.70±6.03 | 0.127 |
| Controls | PCOS | |||
|---|---|---|---|---|
| Parasympathetic reactivity | Sympathetic reactivity | Parasympathetic reactivity | Sympathetic reactivity | |
| Normal | 44 (100%) | 30 (68.2%) | 32 (72.7%) | 10 (22.7%) |
| Early dysfunction | 0 (0%) | 14 (31.8%) | 11 (25%) | 25 (56.8%) |
| Definite dysfunction | 0 (0%) | 0 (0%) | 1 (2.3%) | 9 (20.5%) |
PCOS: Polycystic ovarian syndrome
Normal = all test normal or one test borderline.
Early cardiac autonomic neuropathy= one test abnormal or two test borderline.
Definite cardiac autonomic neuropathy = two tests abnormal.[16]
Classification of values as normal, borderline, or abnormal is given in [Table 1].[16] The result of hs-CRP is summarized in [Figure 1]. The hs-CRP levels were higher in the overweight PCOS group, which was statistically significant from the overweight control group (P = 0.039). The hs-CRP values showed significant positive correlation with SBP (r = 0.307, P = 0.004) and DBP (r = 0.270, P = 0.011) and significant negative correlation with ΔHR component of deep breathing test (DBT) (r = −0.219, P = 0.040) but did not show any correlation with other sympathetic or parasympathetic reactivity tests [Table 3]. This could imply that a greater degree of inflammation was correlated with a decrease in parasympathetic reactivity as DBT is a parasympathetic reactivity test.
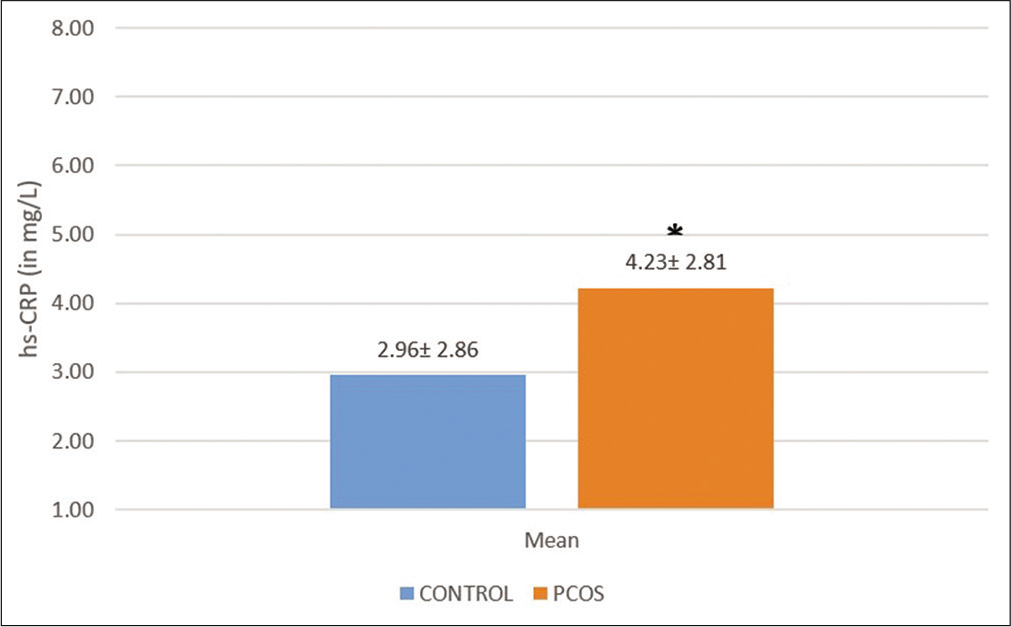
- Comparison of mean high-sensitivity C-reactive protein between overweight polycystic ovarian syndrome patients and overweight control group. The values show mean ± SD. *P-value=0.039.
| Parameters | hs-CRP | |
|---|---|---|
| r | P | |
| SBP | 0.307 | 0.004 |
| DBP | 0.270 | 0.011 |
| Average heart rate | −0.176 | 0.102 |
| E:I ratio | −0.146 | 0.174 |
| Delta heart rate | −0.219 | 0.040 |
| Valsalva ratio | −0.158 | 0.142 |
| 30:15 ratio (LST) | −0.140 | 0.193 |
| IHT (HGT) | −0.148 | 0.169 |
| CPT | −0.139 | 0.196 |
| delta SBP (LST) | 0.144 | 0.181 |
P=0.05 not significant. SBP and DBP show positive correlation with AFT parameters and delta HR shows negative correlation with AFT parameters. SBP: Systolic blood pressure, DBP: Diastolic blood pressure, CPT: Cold pressor test, hs-CRP: High-sensitivity C-reactive protein
DISCUSSION
We found that in the overweight PCOS group, the sympathetic reactivity was significantly less than in the overweight control group. Although the mean values of the parasympathetic reactivity tests were normal for both the groups, the values in the PCOS group were less than that of the control group. Among the parameters which showed significant difference between two groups, VR was less in the PCOS group, as seen in the study by Zanella in PCOS patients of mean BMI 24.25 kg/m2 and BMI-matched controls.[4] The difference in 30:15 ratio was significantly less in the PCOS group which is in agreement with the findings of Saranya et al.[2]
AIIMS AFT lab criteria showed that autonomic dysfunction was more common and severe in the overweight PCOS group as compared to the overweight control group. There were more number of patients with a deranged sympathetic reactivity than deranged parasympathetic reactivity probably due to the fact that sympathetic noradrenergic innervation predominates in all immune organs and sympathetic nerve signaling coordinate vascular, stromal, and immune cell interactions. Hence, its derangement may lead to impaired homeostasis as seen in inflammation.[17]
As most of the patients of PCOS are generally obese, obesity may act as a confounding factor in the autonomic derangements seen in PCOS. If high BMI is the only contributing factor in the abnormalities caused in PCOS, there should not be any significant difference in the case and control groups as both the groups are BMI matched.
The presence of any significant difference calls for additional factors responsible for the autonomic instability.
In other words, obesity may not be the only factor responsible for the deranged parameters seen in PCOS. Hence, other etiological factors may be sought after. Thus along with standard management practices of PCOS which include obesity control and drugs, a regular monitoring of AFT can be very useful in predicting cardiovascular risk to these patients.
A study by Oh et al. suggested that PCOS is not associated with increased CRP levels, but Kelly et al. reported that PCOS patients have significantly higher CRP concentrations than those who had normal menstrual cycles and normal androgen levels.[8,18] A study by Boulman et al. revealed that the mean concentration of CRP was significantly higher in the PCOS subgroups at normal BMI.[12] Moran’s et al. study showed that in overweight PCOS patients, a weight loss of >5 kg accompanied by a decreased CRP and the change in CRP with weight loss correlated with the change in weight.[19]
In our study, the difference between hs-CRP in controls and PCOS was significant (P = 0.039). A higher mean hsCRP value in PCOS group reflected a greater degree of inflammation and increased probability of being associated with greater cardiovascular risk.[20]
As the hs-CRP values showed significant positive correlation with SBP and DBP and significant negative correlation with ΔHR component of DBT, it could imply that a greater degree of inflammation was found in individuals with a lesser value of parasympathetic reactivity. This is in accordance with the concept of presence of neuroimmunomodulation loop, also called cholinergic anti-inflammatory pathway which shows cholinergic signaling interacting with systemic and local inflammation, particularly suppressing immune cells function.[21]
The limitation of our study was that it was done only in the overweight category of BMI. Studies done on all BMI groups, including underweight, normal, overweight, and obese subjects would give a better comparison.
CONCLUSION
Patients with PCOS irrespective of their BMI status are prone to cardiac dysautonomia and non-invasive autonomic function tests may serve as predictors of this. The dysautonomia in PCOS cannot solely be attributed to high BMI factor, hence, weight reduction alone may not be able to improve the autonomic status of the patient.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Heart rate variability as a method of assessing the autonomic nervous system in polycystic ovary syndrome. Rev Bras Ginecol Obstet. 2013;35:421-6.
- [Google Scholar]
- Assessment of cardiovascular autonomic function in patients with polycystic ovary syndrome. J Obstet Gynaecol Res. 2014;40:192-9.
- [CrossRef] [PubMed] [Google Scholar]
- Autonomic dysfunction in patients with polycystic ovary syndrome. Taiwan J Obstet Gynecol. 2015;54:381-4.
- [CrossRef] [PubMed] [Google Scholar]
- Autonomic dysfunction and flow-mediated dilation in polycystic ovary syndrome (PCOS): A case-control study.Dysautonomia in polycistic ovary syndrome. Int J Gynaecol Obstet. 2016;28:19-26.
- [Google Scholar]
- Autonomic dysfunction in women with polycystic ovarian syndrome. Iran J Reprod Med. 2015;13:27-34.
- [Google Scholar]
- Altered autonomic neural control of the cardiovascular system in patients with polycystic ovary syndrome. Int J Cardiol. 2008;130:49-55.
- [CrossRef] [PubMed] [Google Scholar]
- Sympathetic neural overdrive in the obese and overweight state. Hypertension. 2019;74:349-58.
- [CrossRef] [PubMed] [Google Scholar]
- Low grade chronic inflammation in women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2001;86:2453-5.
- [CrossRef] [PubMed] [Google Scholar]
- Endothelial dysfunction in young women with polycystic ovary syndrome: Relationship with insulin resistance and low-grade chronic inflammation. J Clin Endocrinol Metab. 2004;89:5592-6.
- [CrossRef] [PubMed] [Google Scholar]
- C-reactive protein and homocysteine levels are associated with abnormal heart rate recovery in women with polycystic ovary syndrome. Fertil Steril. 2010;94:230-5.
- [CrossRef] [PubMed] [Google Scholar]
- C-reactive protein in healthy subjects: Associations with obesity, insulin resistance, and endothelial dysfunction: A potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;4:972-8.
- [CrossRef] [PubMed] [Google Scholar]
- Increased C-reactive protein levels in the polycystic ovary syndrome: A marker of cardiovascular disease. J Clin Endocrinol Metab. 2004;89:2160-5.
- [CrossRef] [PubMed] [Google Scholar]
- Endothelial dysfunction in PCOS: Role of obesity and adipose hormones. Am J Med. 2006;19:3561-6.
- [CrossRef] [PubMed] [Google Scholar]
- Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41-7.
- [CrossRef] [PubMed] [Google Scholar]
- Methods of evaluation of autonomic nervous system function. Arch Med Sci. 2010;6:11-8.
- [CrossRef] [PubMed] [Google Scholar]
- Pattern and prevalence of cardiovascular autonomic neuropathy in diabetics visiting a tertiary care referral center in India. Indian J Physiol Pharmacol. 2011;55:119-27.
- [Google Scholar]
- Innervation of the immune system In: Kusnecow AW, Anisman H, eds. The Wiley-Blackwell Handbook of Psychoneuroimmunology. New York: John Wiley & Sons Ltd.; 2013. p. :24-49.
- [CrossRef] [Google Scholar]
- Serum C-reactive protein levels in normal-weight polycystic ovary syndrome. Korean J Intern Med. 2009;24:350-5.
- [CrossRef] [PubMed] [Google Scholar]
- C-reactive protein before and after weight loss in overweight women with and without polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:2944-51.
- [CrossRef] [Google Scholar]
- Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular event. N Engl J Med. 2002;347:1557-65.
- [CrossRef] [PubMed] [Google Scholar]
- Sympathetic nervous system and inflammation: A conceptual view. Auton Neurosci. 2014;182:4-14.
- [CrossRef] [Google Scholar]






