Translate this page into:
Multimodal intraoperative neurophysiological monitoring with special emphasis on facial MEPs for facial nerve preservation in vestibular schwannoma surgeries: Surgical nuances and outcome predictability
*Corresponding author: Vamsi Krishna Yerramneni, Department of Neurosurgery, NIMS, Punjagutta, Hyderabad, Telangana, India. vamsiky.ns@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Karanth VK, Yerragunta T, Sampath N, Yerramneni V, Kanala R, Kumar EP, et al. Multimodal intraoperative neurophysiological monitoring with special emphasis on facial MEPs for facial nerve preservation in vestibular schwannoma surgeries: Surgical nuances and outcome predictability. Indian J Physiol Pharmacol. 2024;68:118-25. doi: 10.25259/IJPP_77_2024
Abstract
Objectives:
The objective of this study was to evaluate the utility of facial motor-evoked potentials (FMEPs) as a significant part of multimodal intraoperative neurophysiological monitoring (IONM) for preserving facial nerve (FN) function in vestibular schwannoma surgery guiding the resection, and predicting outcome.
Materials and Methods:
This prospective observational study included 35 cases of vestibular schwannomas operated under multimodal IONM using free-running electromyography (EMG), triggered EMG (with both monopolar probe and suction stimulator), FMEPs, electroencephalography and its spectral derivatives and train-of-four testing. Direct stimulation using a monopolar probe helped in the identification of FN and guiding tumour resection. The suction stimulator probe was used for quasi-continuous stimulus delivery and FN mapping. FMEPs helped in the assessment of the integrity of FN.
Results:
In 14 cases, there was a single instance, and in 11 cases, there was more than one instance of a significant drop in FMEPs. Sixteen cases had >50% fall in FMEPs during surgery. In 28 cases, the proximal stimulation threshold for FN was ≤0.1 mA at the end of the tumour resection. At the end of the first week following surgery, only 42.9% of the cases had good functional preservation (House–Brackmann [HB] Grade I or II) of FN, which increased to 78.8% at the end of one year. The remaining 21.2% had HB Grade III weakness. Percentage drop in FMEP amplitude and final FMEP amplitude correlated significantly (P < 0.01) with the post-operative HB Grade at 1st week, 3, 6 and 12 months following surgery.
Conclusion:
FMEPs, as a significant component of multimodal IONM, provide a real-time assessment of FN function during surgery, facilitate safe maximal resection, predict immediate post-operative FN outcomes, and improve long-term FN function by minimising the cumulative insult inflicted on the FN during surgery.
Keywords
Multimodal intraoperative neurophysiological monitoring
Vestibular schwannoma
Facial nerve
Facial motor-evoked potentials
INTRODUCTION
The goals of Vestibular Schwannoma (VS) surgery are complete tumour excision, avoiding the major neurological deficits, facial nerve (FN) and hearing preservation.[1] The evolution in techniques of micro-neurosurgical resection has achieved the first two goals, but preserving facial functions and hearing is very challenging.[1-3] In the context of large tumours without serviceable hearing but minimal or no FN involvement, which is the usual scenario, the main focus of the surgery shifts toward FN preservation, which will be one of the key patient expectations from the surgeon in terms of cosmesis and protection of cornea.
With the advancement of technology, the surgeon’s armamentarium has also expanded, which includes tools like pre-operative imaging of FN using fast imaging employing steady-state acquisition or diffusion tensor imaging,[4,5] intraoperative neurophysiological monitoring (IONM)[6-10] and neuronavigation.[11] While each technique has its unique advantages and limitations, IONM stands ahead of all for safe maximal resection by its ease of use, real-time feedback, good reliability, predictability and cost-effectiveness.
The aim of this study is to assess FN preservation using multimodal IONM with special emphasis on corticobulbar facial motor-evoked potentials (facial CoMEPs/FMEPs) and surgical nuances for complete excision while attempting to retain the anatomical and functional integrity of FN. This study aims to fill the critical gap in the literature as the utilisation of CoMEPs/FMEPs remains sparse even among developed nations due to the requisite collaboration of skilled personnel, including clinical neurophysiologist, neurosurgeon, and anaesthesiologist, and thereby contributes to both the clinical care and research paradigms.
MATERIALS AND METHODS
This prospective observational study was conducted on 35 cases of VSs operated in our tertiary care hospital between March 2020 and December 2021. Magnetic resonance imaging (MRI) T1-weighted (T1w), T2w, and T1w with contrast were done preoperatively, and all patients underwent audiometric examination. Patients with increased intracranial pressure (ICP) underwent cerebrospinal fluid (CSF) diversion (ventriculoperitoneal shunt) before tumour resection.
Inclusion and exclusion criteria
All VSs larger than 2 cm who were operated on for the first time, irrespective of NF2 status and radiological subtypes, were included in the study. In recurrent cases, those who were not willing to participate and those who had received previous radiotherapy were excluded from the study.
Anaesthesia and positioning
Anaesthetic considerations were taken, aiming at the use of drugs to not interfere with IONM techniques. Total intravenous anaesthesia was used with propofol and fentanyl combination. Muscle relaxant (short-acting) was used only during intubation. Field blocks using local anaesthetics around the FN or muscle were avoided as they could induce temporary paresis and confound the electrophysiological changes in IONM. Local anaesthetics near the stylomastoid foramen were also avoided. All of the patients were operated on in a supine position with their necks turned toward the opposite side of the tumour.
IONM
IONM was done using free-running electromyography (EMG), triggered EMG (using both monopolar flush tip stimulator and suction stimulator), FMEPs, electroencephalography (EEG) and its spectral derivatives and train-of-four testing. The terminal branches of the FN were monitored using subdermal paired-pin braided electrodes placed in the muscles, namely, frontalis, orbicularis oculi, nasalis, orbicularis oris and mentalis. Mandibular division of the trigeminal nerve was also monitored using a subdermal electrode placed in the masseter.
We used corkscrew electrodes for corticobulbar FMEP stimulation in Cz (cathode) – C3/C4 (anode) or Mz (cathode) – M3/M4 (anode) for hemispheric stimulation. The stimulation parameters were titrated in the following range: intensity was between 100V and 400V or 40 and 150 mA; a train of 3–8 rectangular pulses was used with a pulse width ranging from 75 us to 500 us, and the interpulse interval was in the range of 1.5–3 ms. This train stimulus was followed by a single pulse of the same characteristic delivered 50–90 ms later to differentiate true CoMEP from a peripheral response.
Direct stimulation using a flush-tip monopolar stimulator probe (Medtronic, USA) helped in the identification of FN and guiding tumour resection. A suction stimulator probe (Inomed, Germany) was used for quasi-continuous stimulus delivery and FN mapping. A single pulse of cathodal polarity with a width ranging from 200 to 500 us was used for triggered EMG, and it was delivered at a rate of 3 Hz for both monopolar flush tip and suction probe stimulation with a maximal intensity not exceeding 4 mA.
FMEPs helped in the assessment of the integrity of FN at any given instance. All consistent changes in FMEP and persistent neurotonic discharges were conveyed to the surgical team. A drop in FMEP amplitude of >50% constituted an alert.
Train-of-four was used to assess the residual neuromuscular blockade effect following the initial dose of relaxant administration during intubation. EEG and spectral derivatives of EEG were used to assess the depth of anaesthesia. Representative graphs of an illustrative case is demonstrated in Figures 1-4.
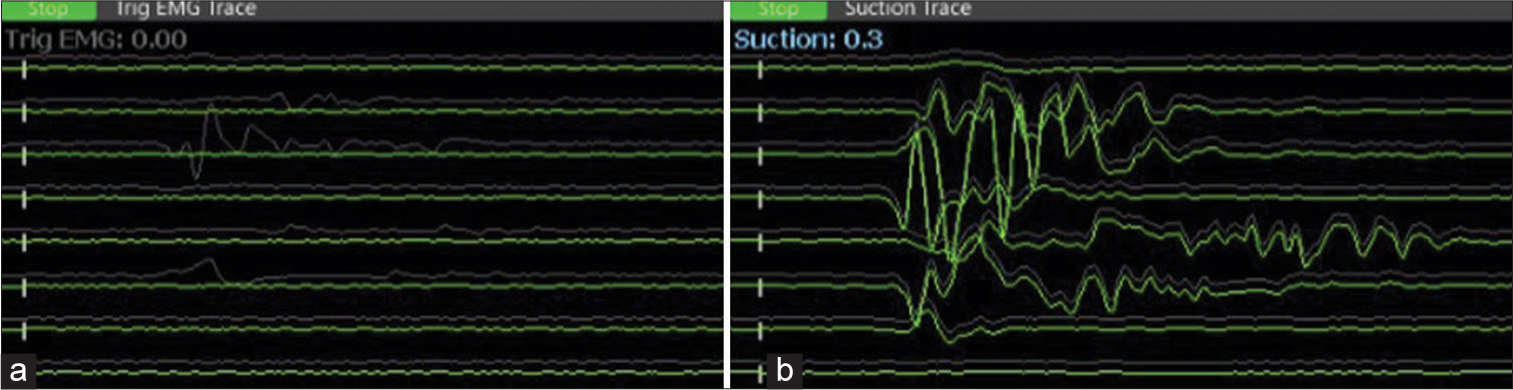
- (a) The monopolar stimulator was not used concomitantly, as can be seen on the left side panel. (b) The surgeon is alerted to the proximity of the facial nerve by suction stimulator response at a threshold of 0.3 mA (right panel). (Channels from top to bottom: frontalis, oculi_1, oculi_2, nasalis, oris_upperlip, oris_lowerlip, masseter_1, masseter_2).
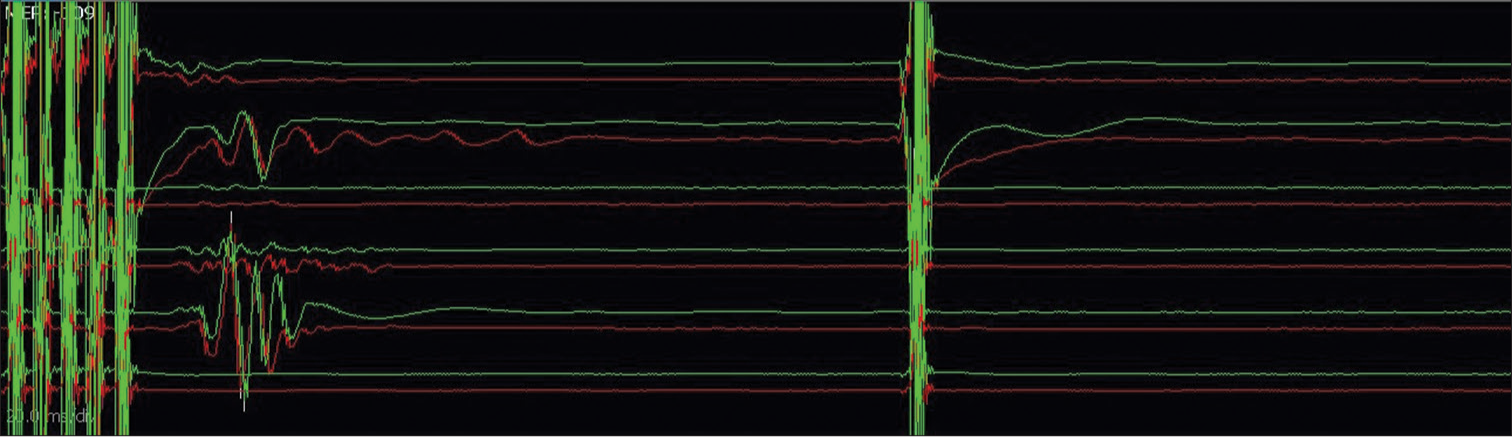
- Double-train technique for establishing true corticobulbar facial motor-evoked potentials – responses noted only following train stimulation and not after a single pulse stimulation (Channels from top to bottom: frontalis, oculi_1, oculi_2, oris_upperlip, oris_lowerlip, masseter).
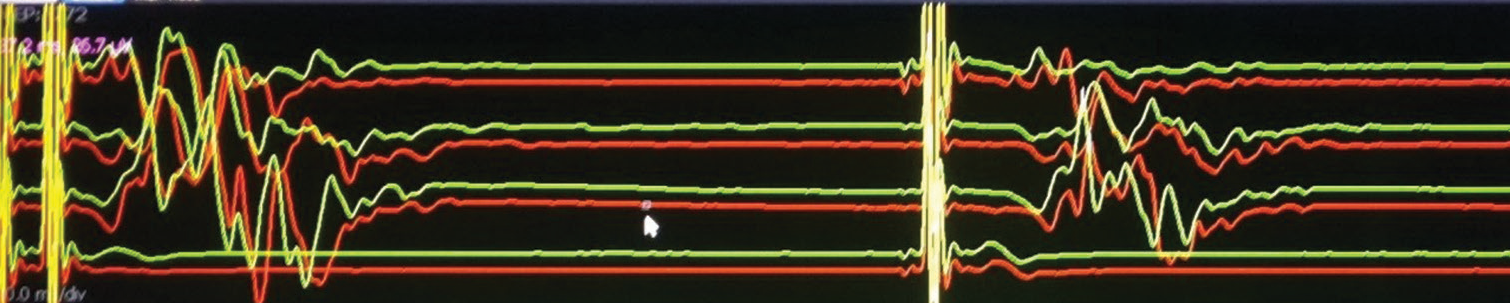
- Contaminated facial responses due to direct activation of the peripheral facial nerve bypassing the corticobulbar pathway – as responses are noted following single pulse stimulation (Channels from top to bottom: frontalis, oculi, oris, masseter).
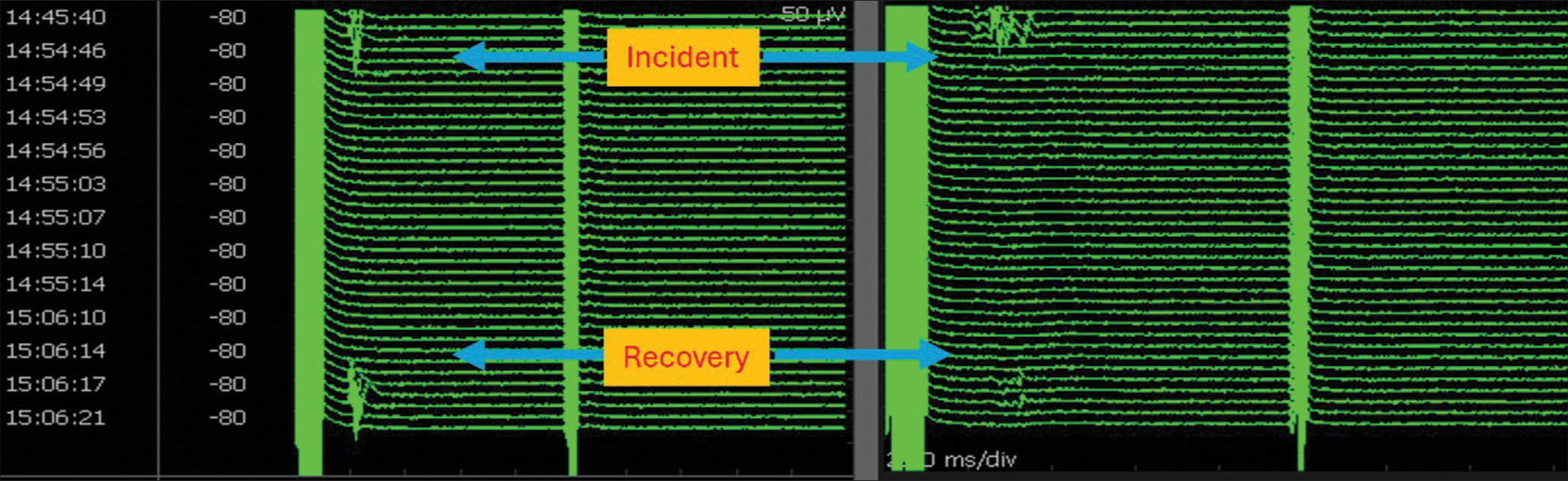
- Stack view of facial motor-evoked potentials (FMEP) drop followed by recovery following bipolar cautery to arrest bleeding: illustrated channels are oculi (left) and oris (right). There were no concomitant neurotonic discharges. Note from the timestamp that it took about 12 min for the FMEP drop to recover. During this time, surgical pause was initiated, blood pressure was raised, and papaverine was irrigated. This is a classical illustration of the identification of an ongoing facial nerve injury at an early reversible stage using FMEPs and initiation of corrective measures resulting in near-total recovery. Surgery was resumed following recovery.
Surgical procedure
Classic retromastoid suboccipital craniotomy was done. A durotomy was done, and CSF was let out from the cerebellopontine (CP) angle cistern. The tumour was identified, baseline FMEPs were established, and the tumour capsule was stimulated with a monopolar probe to map the FN. Monopolar stimulation threshold intensity of <0.1 mA would indicate that the probe is on the nerve; a higher intensity of up to 1 mA would be required to stimulate an injured FN; higher stimulation intensities would also be required when testing through the bone (0.5–2 mA) or soft/tumour tissue (0.3–1 mA).[12] The presence of fluid causes shunting of current, and it can produce a false-negative response. Hence, the use of stimulation threshold to guide resection is highly contextual and requires a good understanding between the surgical and the neurophysiologist team.
A simple heuristic that we practice is as follows: stimulation at currents lower than 0.2 mA indicates close proximity of the nerve to the probe, and it may be covered by a thin layer of tumour, which is at risk of injury if manipulated.[13,14] If there is a thick tumour covering the nerve, stimulation thresholds shall be at currents >0.5 mA.[14] Highly adherent tumours also need higher stimulation thresholds as they create thicker barriers.[15] However, as has already been explained, this current-distance relationship is not so simple and depends on multiple factors.
The surgery proceeded with central debulking using an ultrasonic aspirator. The capsule of the tumour is dissected from the arachnoid plane and rolled inwards. Every time the part to be decompressed is swept with the suction stimulator; hence, the cycle of stimulation, decompression and mobilisation is repeated many times to thin out the tumour.
After achieving approximately more than half of the tumour resection, internal auditory canal (IAC) drilling was done, and tumour decompression in IAC was done while intermittently mapping the nerve with the monopolar stimulator. FN is identified at its proximal end at the brainstem and distal end at the IAC. If FN dissection at any point along its course was found difficult due to dense adhesion, part of the tumour capsule was left behind after adequately thinning it with the ultrasonic aspirator.
FMEPs were recorded at regular intervals and every time before changing the direction of dissection and before and after any coagulation when approaching the FN response zone. Any fall in the amplitude was notified, and, in such cases, the surgical field was irrigated with saline and diluted papaverine; blood pressure was raised, and surgery was paused to facilitate recovery of FMEPs, usually for a maximum of 15–30 min.
Whenever the electrophysiology team alerts the surgeon about high frequency neurotonic discharges, tumour manipulation was briefly paused to let the discharges subside, and papaverine and saline irrigation was done, if persistent.
An anatomical and functional preservation of the FN was confirmed at the end of the surgery by checking FMEPs and stimulating the nerve at the brainstem end. If the FMEP at closure was at least 50% of baseline response, we expect the functional integrity of the FN, while an FMEP amplitude drop of > 50% would predict an immediate mild-to-moderate post-operative facial weakness and if there was no response, a severe injury, which was predicted with dense facial palsy. Proximal stimulation threshold 0.05–0.1 mA would indicate good functional preservation[16-18], whereas 0.2–1 mA would indicate some damage, at least in the immediate postoperative period, which may be due to temporary stunning (neuropraxia), which does not reflect the long-term outcome.[14,19-23]
Inadvertent injury causing a breach in the anatomical integrity of FN was identified using monopolar stimulation and inspection of the course of the nerve. In such cases, it was sutured using 10-0 nylon and the anastomosis was stabilised with the use of thrombin glue Tisseel (Baxter International Inc., Westlake Village, CA, USA).
Post-operative care
After recovery, the FN was examined and compared with the opposite side and pre-operative status. Post-operative MRI was done three months following the surgery. The resection extent was considered gross-total resection (GTR) when no tumour was left, near-total resection when a thin layer of the tumour was left on the FN to prevent its injury, and subtotal resection when a significant tumour was left in the CP angle cistern. All the patients were followed up for a minimum of one year.
Statistical analysis
IBM Statistical Package for the Social Sciences Statistics 26 was used for analysing the data. Mean values were used to describe continuous variables, and percentages were used for categorical variables. A paired t-test was applied for continuous data. Pearson correlation test was used for the analysis of correlation in continuous data, and Spearman’s rho test was used for analysing the correlation when at least one parameter was ordinal. P ≤ 0.05 was considered statistically significant.
RESULTS
Thirty-five cases of VSs were operated under IONM, including 14 males and 21 females. The mean age was 41 years, and the majority of the patients had hearing loss, tinnitus, vestibular and cerebellar symptoms. Raised ICP was present in 12 patients, and they were treated with CSF diversion. All the patients had non-serviceable hearing. FN was completely preserved in 19 patients (House–Brackmann [HB] grade I), and 16 patients had mild FN palsy (HB grade II) preoperatively. Six cases had moderate size tumours, and the remaining 29 cases had large (>2.5 cm) tumours, and the average tumour size was 3.74 cm. Gross total excision was achieved in 29 cases; five cases had near-total excision, and one patient had subtotal excision. All the patients had a minimum of 1-year follow-up and two cases deceased (one – post-operative meningitis and sepsis, one – hypertensive haemorrhage and unrelated to surgery) during the follow-up period [Table 1].
| Groups | Number of cases |
|---|---|
| Age | |
| <20 years | 2 |
| 20–40 years | 15 |
| 40–60 years | 14 |
| >60 years | 4 |
| Gender | |
| Male | 14 |
| Female | 21 |
| Tumour size | |
| 2–2.5 cm (Medium) | 4 |
| 2.5–4 cm (Large) | 18 |
| >4 cm (Giant) | 13 |
| Pre-operative HB grade | |
| 1 | 19 |
| 2 | 16 |
| Excision type | |
| GTE | 29 |
| NTE | 5 |
| STE | 1 |
| Nerve injury | |
| No | 33 |
| Yes | 2 |
| Follow-up | |
| 12 months | 33 |
| Deceased | 2 |
GTE: Gross total excision, NTE: Near-total excision, STE: Subtotal excision, HB: House-Brackmann
In 14 cases, there was a single instance, and in 11 cases, there was more than one instance of a significant drop in FMEPs. Persistent neurotonic discharges were noted in almost all cases when working close to the nerve. Sixteen cases had >50% fall in motor-evoked potentials (MEPs) during surgery, which recovered in a few minutes (3–30 min) with interventions. Ten cases had <50% of baseline MEPs at the end of the surgery, which included five cases where there was complete loss (flat MEPs). In 28 cases, the FN proximal stimulation threshold was ≤0.1 mA at the end of the surgery. Four cases required a higher threshold (>1 mA), and in three cases nerve was not excitable [Table 2].
| Groups | Number of cases |
|---|---|
| Number of instances of drop | |
| No drop | 10 |
| 1 | 14 |
| 2 | 10 |
| 3 | 1 |
| MEP drop in percentage | |
| <50%/No drop | 14 |
| 50–100% | 13 |
| 100% | 6 |
| Final MEP in % | |
| 100% | 17 |
| 50–100% | 8 |
| <50%/Flat MEP | 8 |
| Final stimulus threshold in mA | |
| <0.1 mA | 17 |
| 0.1–1 mA | 10 |
| >1 mA/Not stimulated | 6 |
| Post-op HB grade | |
| D1 | |
| Grade I or II | 23 |
| HB Grade III | 12 |
| Grade IV | - |
| 1 Week | |
| Grade I or II | 15 |
| HB Grade III | 13 |
| Grade IV | 7 |
| 3 Months | |
| Grade I or II | 22 |
| HB Grade III | 11 |
| Grade IV | - |
| 6 Months | |
| Grade I or II | 26 |
| HB Grade III | 7 |
| Grade IV | - |
| 12 Months | |
| Grade I or II | 26 |
| HB Grade III | 7 |
| Grade IV | - |
IONM: Intraoperative neurophysiological monitoring, MEP: Motor-evoked potential, HB: House–Brackmann
At the end of 1st week following surgery, 42.9% (n = 15) of the cases had good functional preservation (HB Grade I or II) of FN, and 37.1% (n = 13) of the cases had HB Grade III, and 20% (n = 7) had Grade IV weakness. There was a significant improvement in FN function in the first 3 months following surgery, and the percentage of good functional preservation (HB Grade I or II) had increased to 66.7%. At the end of 1 year, 78.8% of the operated cases had good functional preservation and remaining had HB Grade III weakness, and none of the patients had FN weakness of HB Grade IV or more [Table 2].
Percentage drop in MEP amplitude and final MEP amplitude correlated significantly (P < 0.01) with the post-operative HB Grade at 1st week 3, 6 and 12 months following surgery [Table 3]. The final proximal stimulation threshold only correlated with the HB Grade at 1st post-operative week (P = 0.023).
| MEP Drop in % | Final MEP | Final Stimulus Threshold | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Post-operative HB Grade at 12 months | 100% | 50-100% | <50%/No drop | 100% | 50–100% | <50%/Flat MEP | <0.1 mA | 0.1–1 mA | >1 mA/Not Stimulated |
| Number of cases | 6 | 13 | 14 | 17 | 8 | 8 | 17 | 10 | 6 |
| Grade I, II FN weakness (%) | 2 (33.3) | 10 (77) | 14 (100) | 17 (100) | 7 (87.5) | 2 (25) | 17 (100) | 7 (70) | 2 (33.3) |
| Grade III FN weakness (%) | 4 (66.7) | 3 (23) | 1 (12.5) | 6 (75) | 3 (30) | 4 (66.7) | |||
IONM: Intraoperative neurophysiological monitoring, MEP: Motor-evoked potential, HB: House–Brackmann, FN: Facial nerve
DISCUSSION
In the contemporary neurosurgical practice, the use of IONM for FN preservation is considered as the standard of care, as few historical case series,[8,24,25] have shown a clear benefit. Many patients present to us with very large tumours and non-serviceable hearing but preserved FN function. They are at higher risk of post-operative FN deficits.[16,26-28]
There is plenty of literature which discusses FN preservation in VS surgery with varied results. A recent meta-analysis[29] summarised many studies and has thrown light on many salient aspects. A broad range (30–84%) of good FN outcomes (HB grade I and II) was noted, which was mainly attributed to the surgeon’s experience. On average, 60.1% is the FN preservation rate in a series of large VSs.[29]
Although it is difficult to generalise the findings of our small series, we could achieve a slightly better outcome (78.8% vs. 60.1%) with the use of multimodal IONM. The GTRs tend to be associated with temporary higher-grade weakness, which improves over a period of 6 months, provided FMEPs are preserved intraoperatively.
Prediction of post-operative FN outcome using IONM
The role of IONM has grown from an identification tool for mapping the nerve during surgery to an indicator of FN functional preservation and predictor of outcome.[24,25] Several studies have correlated the intraoperative parameters such as A-train neurotonic discharges, stimulus threshold, response amplitude and proximal to distal amplitude ratio with FN outcome.
Post-resection stimulation thresholds at the root entry zone and response amplitudes have proven to predict good functional outcomes and have been used as prognostic indicators.[24,25] In our series, seven cases who had stimulus threshold >1 mA (or nerve was not excitable) at the end of the surgery had higher grade (HB Grade III or IV) weakness at 3 months.
Tawfik et al.[30] defined degradation of FMEP response as a final-to-baseline amplitude ratio of 0.5 or less and found that FMEP has high specificity (88.9%) and moderate sensitivity (54.5%) for predicting immediate post-operative function. Ling et al.[31] also found that the FMEP amplitude ratio significantly correlated with short-term and long-term postoperative FN functions. Machetanz et al. have noted different sensitivity and specificity of FMEP and EMG in predicting post-operative FN palsy, and their combined use was found to be more accurate.[32]
FMEPs are of major use in large VS, where the proximal part of FN will be identified at a much later stage of the surgery.[33] Response amplitude of the FMEPs at the end of resection correlated with its function postoperatively. In our series, more than 50% fall in FMEPs correlated well with temporary higher grade facial palsy.
In our series, five cases had absence of electrophysiological responses (flat MEPs) at the end of the surgery and HB grade IV weakness at the 1st week; they improved to Grade III in 3 months, hence in an anatomically intact FN, the absence of electrophysiological responses or spontaneous tonic/train activity cannot be considered as definite indicator of permanent FN paralysis as it may indicate temporary stunning of the FN conduction due to neuropraxia.[34]
Drugs in outcome improvement
Perioperative steroids were used to control oedema and nerve damage. Papaverine, a vasodilator used during surgery, when we have fall in FMEPs, helps in relieving vasospasm. Nimodipine, a calcium channel blocker, helps in resprouting, growth of axons and remyelination.[35]
Neurotisation in outcome improvement
With the use of IONM, we can identify the FN injury and do the neurotisation. The presence of response distal to the site of injury and its absence on proximal stimulation helps to localise the site of transection of FN. Following neurotisation, there may not be immediate recovery of the FMEPs, and there can be temporary high-grade palsy, but there will be a long-term improvement in FN function.
Another observation made in our study is that the use of thrombin glue (Tisseal) for stabilising neurotisation helps in the early recovery of FN function. This could be attributed to fibrin, which may help to align and stabilise the anastomosis, or calcium chloride, which could help in nerve conduction. However, this needs further evaluation to prove its efficacy, as it was based on only two case observations, and we have not done statistical analysis.
CONCLUSION
FMEPs, as a significant component of multimodal IONM, are reliable in providing a real-time assessment of FN function during surgery. This provides live feedback to the operating surgeon to minimise intraoperative neural damage, thus facilitating safe maximal resection and predicting immediate post-operative FN outcomes. The use of multimodal IONM improves long-term FN functional status by minimising the cumulative insult inflicted on the FN throughout surgery. They are also useful in refining the surgical techniques of tumour resection and achieving GTR in the majority of the patients.
Acknowledgement
The authors thank patients, institute, medical and paramedical staff for their direct and indirect contribution.
Ethical approval
The research/study was approved by the Institutional Review Board, number NIMS/ISRC/106/2014 dated 25/03/2024.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Individualizing hearing preservation in acoustic neuroma surgery. Laryngoscope. 1997;107:1043-7.
- [CrossRef] [PubMed] [Google Scholar]
- Neural conservation in skull base surgery. Otolaryngol Clin North Am. 2002;35:411-24, ix
- [CrossRef] [PubMed] [Google Scholar]
- Hearing preservation in vestibular schwannoma surgery: What factors influence outcome? J Neurosurg. 1995;83:191-6.
- [CrossRef] [PubMed] [Google Scholar]
- Preoperative diffusion tensor imaging-fiber tracking for facial nerve identification in vestibular schwannoma: A systematic review on its evolution and current status with a pooled data analysis of surgical concordance rates. Neurosurg Focus. 2018;44:E5.
- [CrossRef] [PubMed] [Google Scholar]
- Congress of neurological surgeons systematic review and evidence-based guidelines on the treatment of adults with vestibular schwannomas: Executive summary. Neurosurgery. 2018;82:129-34.
- [CrossRef] [PubMed] [Google Scholar]
- A comparison of facial nerve monitoring systems in cerebellopontine angle surgery. Am J Otol. 1991;12:1-6.
- [Google Scholar]
- Improved preservation of facial nerve function with use of electrical monitoring during removal of acoustic neuromas. Mayo Clin Proc. 1987;62:92-102.
- [CrossRef] [PubMed] [Google Scholar]
- Facial nerve monitoring in acoustic tumor surgery. Otolaryngol--Head Neck Surg. 1991;104:814-7.
- [CrossRef] [PubMed] [Google Scholar]
- Facial nerve monitoring in skull base surgery. J Laryngol Otol. 1994;108:557-9.
- [CrossRef] [PubMed] [Google Scholar]
- Acoustic (loudspeaker) facial electromyographic monitoring: Part 1. Evoked electromyographic activity during acoustic neuroma resection. Neurosurgery. 1986;19:392-400.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of the facial nerve in relation to vestibular schwannoma using preoperative diffusion tensor tractography and intraoperative tractography-integrated neuronavigation system. World Neurosurg. 2017;107:669-77.
- [CrossRef] [PubMed] [Google Scholar]
- Best practices in facial nerve monitoring. Laryngoscope. 2021;131:S1-42.
- [CrossRef] [PubMed] [Google Scholar]
- Intraoperative monitoring of facila muscle evoked responses obtained by intracranial stimulation of the facila nerve: A more accurate technique for facila nerve dissection. Neurosurgery. 1979;4:418-21.
- [CrossRef] [PubMed] [Google Scholar]
- Routine intraoperative facial nerve monitoring during otologic surgery. Am J Otol. 1988;9:269-75.
- [Google Scholar]
- Comparison between intraoperative observations and electromyographic monitoring data for facial nerve outcome after vestibular schwannoma surgery. Acta Otolaryngol (Stockh). 2005;125:1069-74.
- [CrossRef] [PubMed] [Google Scholar]
- Predictive factors of long-term facial nerve function after vestibular schwannoma surgery. Otol Neurotol. 2002;23:388-92.
- [CrossRef] [PubMed] [Google Scholar]
- Facial nerve monitoring parameters as a predictor of postoperative facial nerve outcomes after vestibular schwannoma resection. Otol Neurotol. 2005;26:728-32.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of response amplitude versus stimulation threshold in predicting early postoperative facial nerve function after acoustic neuroma resection. Am J Otol. 1998;19:112-7.
- [Google Scholar]
- Facial nerve outcome with a peroperative stimulation threshold under 0.05 mA. Laryngoscope. 2011;121:2295-8.
- [CrossRef] [PubMed] [Google Scholar]
- Value of intraoperative threshold stimulus in predicting postoperative facial nerve function after acoustic tumor resection. Am J Otol. 1997;18:249-51.
- [Google Scholar]
- Predictive value of facial nerve electrophysiologic stimulation thresholds in cerebellopontine-angle surgery. Laryngoscope. 1996;106(5 Pt 1):633-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prediction of facial nerve function following acoustic neuroma resection using intraoperative facial nerve stimulation. Laryngoscope. 1994;104(5 Pt 1):539-44.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic significance of intraoperative facial nerve stimulus thresholds. Am J Otol. 1997;18:494-7.
- [Google Scholar]
- Intraoperative facial nerve monitoring in the surgery of cerebellopontine angle tumors: Improved preservation of nerve function. ORL J Otorhinolaryngol Relat Spec. 1994;56:31-5.
- [CrossRef] [PubMed] [Google Scholar]
- Intraoperative facial nerve monitoring in acoustic neuroma surgery. Am J Otol. 1993;14:524-32.
- [Google Scholar]
- Acoustic neuromas: Results of current surgical management. Neurosurgery. 1997;41:50-8. discussion 58-60
- [CrossRef] [PubMed] [Google Scholar]
- Current results of the surgical management of acoustic neuroma. Skull Base. 2002;12:189-95.
- [CrossRef] [PubMed] [Google Scholar]
- Facial nerve injury in acoustic neuroma (vestibular schwannoma) surgery: Etiology and prevention. J Neurosurg. 1997;87:60-6.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical management for large vestibular schwannomas: A systematic review, meta-analysis, and consensus statement on behalf of the EANS skull base section. Acta Neurochir (Wien). 2020;162:2595-617.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of motor-evoked potential monitoring on facial nerve outcomes after vestibular schwannoma resection. Ann Otol Rhinol Laryngol. 2019;128:56-61.
- [CrossRef] [PubMed] [Google Scholar]
- Predictive value of intraoperative facial motor evoked potentials in vestibular schwannoma surgery under 2 anesthesia protocols. World Neurosurg. 2018;111:e267-76.
- [CrossRef] [PubMed] [Google Scholar]
- Predictive value of facial motor-evoked potential and electromyography for facial motor function in vestibular schwannoma surgery. Acta Neurochir (Wien). 2024;166:23.
- [CrossRef] [PubMed] [Google Scholar]
- Facial nerve monitoring during cerebellopontine angle and skull base tumor surgery: A systematic review from description to current success on function prediction. World Neurosurg. 2013;80:e271-300.
- [CrossRef] [PubMed] [Google Scholar]
- The anatomically intact but electrically unresponsive facial nerve in vestibular schwannoma surgery. Neurosurgery. 2012;71:1125-30. discussion 1130
- [CrossRef] [PubMed] [Google Scholar]
- Nimodipine promotes regeneration and functional recovery after intracranial facial nerve crush. J Comp Neurol. 2001;437:106-17.
- [CrossRef] [PubMed] [Google Scholar]







