Translate this page into:
Intra-arterial injection of bradykinin produces reflex cardiorespiratory changes involving histamine receptors in anesthetized rats
*Corresponding author: Maloy B. Mandal, Department of Physiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India. maloy_mandal@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Singh SK, Mandal MB. Intra-arterial injection of bradykinin produces reflex cardiorespiratory changes involving histamine receptors in anesthetized rats. Indian J Physiol Pharmacol 2020;64(3):174-80.
Abstract
Objectives:
It is well known that intra-arterial injection of nociceptive agent produces vasosensory reflex responses altering cardiorespiratory parameters. The role of various inflammatory mediators is also implicated in the regulation of these reflex responses. However, the role of histamine in this regard is not clear. This study was performed to understand the role of H1 and H2 receptors in modulating the cardiorespiratory responses evoked after i.a. injection of bradykinin (BK).
Materials and Methods:
Male albino rats were anesthetized with an intra-peritoneal injection of urethane (1.5 g/kg). Tracheostomy was performed to keep the respiratory tract patent. The femoral artery was cannulated proximally by pediatric i.v. cannula (24 G, double ported). This cannulation was used for the blood pressure (BP) recording as well as for the drugs instillation as it contains double port with injection valve. The effect of BK (1 µM) on BP, electrocardiographic, and respiration was recorded for 30 min. The respiratory frequency, respiratory minute volume, mean arterial pressure, and heart rate were computed from the original tracings and the data were presented as mean ± SEM.
Results:
Intra-arterial injection of BK produced immediate hyperventilatory (50% from initial), hypotensive (40% from initial), and bradycardiac responses (17% from initial) of shorter latency (5–8 s) indicating the neural mechanisms in producing the responses. Pre-treatment with pheniramine maleate significantly attenuated the BK-induced hyperventilatory (11% from initial), hypotensive (8% from initial), and bradycardiac responses (2% from initial).
Conclusion:
Our data provide evidences for the involvement of H1 and H2 receptors in producing the BK-induced vasosensory reflex responses modulating the cardiovascular parameters in anesthetized rats.
Keywords
Vasosensory reflexes
Nociceptive agents
Bradykinin
Pheniramine maleate
Cardiorespiratory changes
INTRODUCTION
It is well known that the baroreceptors and chemoreceptors present in great blood vessels originating from heart, play an important role in regulation of cardiorespiratory parameters but the role of peripheral blood vessels in this regard has been a matter of discussion.[1,2] Sensory nerves innervating the blood vessels have been associated in the pain of vascular origin such as migraine, angina, embolism, intermittent claudication, and myocardial infarction.[3,4] It has been suggested that the autonomic changes and cardiorespiratory changes seen in vascular disorders are mediated reflexly by the activation of sensors located around the peripheral blood vessels.[5,6] The involvement of vanilloid receptor 1 (VR1) has been associated for the immediate nociceptive responses evoked after intra-arterial (i.a.) injection of capsaicin.[7,8] Further, it is also known that capsaicin-induced responses are short lived (15 s) in comparison to the venom-induced vasosensory reflex responses.[9] The shorter duration of action of capsaicin is attributed to the phenomenon of rapid desensitization produced by it. Intra-arterial injection of Mesobuthus tumulus venom produced long-lasting sequential alterations in cardiorespiratory parameters.[9-11] Involvement of prostaglandins has also been demonstrated for mediating venom-induced vasosensory responses.[10]
Bradykinin (BK) is a potent nociceptive component of the venom; therefore, it was planned to use the BK for the elicitation of the vasosensory reflex responses in this study. It has been shown elsewhere that i.a. injection of BK produced immediate hyperventilatory and hypotensive responses.[12] However, the effect of BK on heart rate (HR) was not assessed in their study. Previously, the role of peripheral blood vessels in regulation of cardiorespiratory parameters has been demonstrated by some workers.[7,8,10] It has also been shown that BK receptors are involved in producing these responses.[12] Since, all the inflammatory mediators (e.g., BK, histamine, and prostaglandins) are well known nociceptive agent; therefore, the role of histamine receptors in mediating the BK-induced responses cannot be ruled out. Therefore, the present study was undertaken to evaluate the role of histamine receptors (H1 and H2 receptors) in producing the BK-induced vasosensory reflex responses.
MATERIALS AND METHODS
All the chemicals (saline/drugs) were instilled in the femoral artery retrogradely and six animals were used in each group. The volume of injectables was kept minimum and constant (0.10 ml) for all chemicals and temperature of the lab was maintained at 25 ± 2°C.
Animals and anesthesia
Ethical clearance was obtained from the Institute Animal Ethical Committee, Institute of Medical Sciences, Banaras Hindu University, Varanasi (Dean/2019IAEC/1627 Dated: 17/11/2019). The studies involving animals described herein were performed in accordance with Guide for Care and Use of Laboratory Animals issued by the Indian National Academy of Science. Animals were housed with 12:12 h light/dark cycle and food (Hindustan Lever Ltd., Mumbai) and water was provided ad libitum. Healthy male albino rats (Charles-Foster strain; 234 ± 9.32 g) were anesthetized with an intra-peritoneal injection of urethane (1.5 g/kg). Urethane (Merck, Germany) was dissolved in double distilled water in the concentration of 0.5 g/ml. A maintenance dose (50–100 mg) of anesthesia was given as per the requirement.
Dissection and recording
Following the standard procedure, tracheostomy was performed to keep the respiratory tract patent. Length of tracheostomy tube was kept short to minimize the dead space. Tracheal secretions were aspirated from time to time. The femoral artery was dissected and isolated in the femoral triangle. Then, it was cannulated proximally by pediatric i.v. cannula with injection valve (24 G, double ported) filled with heparinized saline (20 IU/ml). This cannula was used for the BP recording as well as for the administration of drugs as it contains double port. The single cannulation of femoral artery for BP monitoring and drug administration minimized the surgical injury to the animals. We also avoided the cannulation of common carotid artery as it may compromise the circulation of pontomedullary areas which regulate the cardiorespiratory reflexes. Further, the cannula was connected to a pressure transducer which, in turn, was connected to a bridge amplifier and finally to the data acquisition system (Power Lab 26T, AD Instrument, Australia) to record, displays and analyze the data on the personal computer through Labchart-8 software [Figure 1]. The respiratory movements were recorded by securing the skin over xiphisternum and connecting it to a force transducer through a thread. The electrocardiographic (ECG) potentials were recorded using needle electrodes, connected in standard limb lead-II configuration. The blood pressure (BP), respiratory movements and ECG were recorded on a PC connected to the data acquisition system [Figure 1]. At the end of the experiments, animals were sacrificed by overdose of anesthesia. The deflection of chest wall was then recorded by introducing a known volume (1 ml) of air into the lung through the tracheal tube and was computed as “x.” For calculation of ventilation, the average amplitude (h) of respiratory excursions for a period of 5 s was measured. The height (mm) of respiration was then converted to volume (ml) using the calibration [h/x]. Respiratory minute volume (RMV) was then calculated by multiplying the respiratory frequency (RF) with [h/x].
Drugs and solutions
Urethane was procured from Merck, Germany, and was freshly prepared (0.5 g/ml) in double distilled water before each experiment. BK acetate salt was procured from the Sigma Chemicals Company, St Louis, USA. Stock solution of BK (1 mg/ml) was prepared in double distilled water and was refrigerated. Subsequent dilutions were made in normal saline at the time of experimentation. Volume of all the drugs was kept minimum and constant (0.10 ml) to minimize the stretch-induced responses and systemic spillage. Pheniramine Maleate injection (20 mg/ml) was obtained from the Sanofi India Limited, Vadodara, Gujarat, India. Heparin (1000 IU/ml) was obtained from Biological Evans Ltd., Hyderabad.

- Diagrammatic presentation of the rat showing experimental setting for recording of various cardiorespiratory parameters. TC: Tracheal cannulation, N: Needle secured to skin, FT: Force transducer, FA: Femoral artery, C: Cannula (24G, double ported), I: Instillation port for drugs/saline, BPT: Blood pressure transducer; DAS: Data acquisition system (AD Instruments), LLE: Left leg electrode, RAE: Right arm electrode, ECGE: ECG electrodes, GE: Ground electrode.
Experimental protocol
BK only responses
After surgical procedure, animals were allowed to stabilize for 30 min and the initial recordings of BP, ECG, and respiration were made for 15 min at the interval of 5 min. Equi-volume of normal saline (0.10 ml) was injected in the segment of femoral artery and the observations were made for 15 min at the interval of 5 min. BK (1 μM, 0.10 ml) was injected in the femoral artery retrogradely and the recordings were made for 30 min at every 5 min of interval.
Effect of pheniramine maleate on BK-induced responses
After stabilization of the animal for 30 min, the initial recordings of BP, ECG, and respiration were done. The pheniramine maleate (0.11 mg or 0.45 mg/kg) was injected in the femoral artery and the recordings for the cardiorespiratory parameters were made for the 20 min at the interval of 5 min to see the effect of antagonist alone. BK (1 µM) was injected in the femoral artery in these animals and the cardiorespiratory parameters were recorded for 30 min at the interval of 5 min.
Statistical analysis
The results were presented as mean ± SEM values. The statistical significance between two groups was analyzed by comparing the RF, RMV, mean arterial pressure (MAP), and HR responses of BK only group with the pheniramine pretreated group. The comparisons were made using Student’s t-test for paired and post hoc correction using Dunnett’s t-test (two sided) for unpaired observations by SPSS-16.0 software. P < 0.05 was considered as significant.
RESULTS
It has been observed that i.a. injection of 1 μM concentration of BK (0.10 ml) produced optimal cardiorespiratory responses on all parameters. Therefore, this concentration was used for the elicitation of vasosensory reflex responses.
BK-induced changes on cardiorespiratory parameters
After i.a. injection of BK (1 μM), there was immediate increase in RF (from 86.7 ± 2.67 to 125.3 ± 5. 63/min) and RMV (152.3 ± 8.4 to 298.5 ± 12.01 ml/min). The increase in RF and RMV was significantly greater from the initial value (P < 0.05, Student’s t-test for paired observations). Response was short lived which lasted for few seconds and comes to the initial level within 5 min and subsequently remained at that level up to 30 min [Figures 1and 2].
After injection of BK, there was immediate fall in MAP (from 82 ± 4.15 mmHg to 49 ± 3.67 mmHg). The decrease in MAP was significantly greater from the initial value (P< 0.05, Student’s t-test for paired observations). This hypotensive response was transient which reached the initial level within 5 min and remained at that level [Figures 1and 2].
After injection of BK, there was immediate bradycardiac response (from 321.2 ± 4.22 bpm to 266.7 ± 9.19 bpm). This decrease in HR was significantly greater from the initial value (P < 0.05, Student’s t-test for paired observations) which returns back to the initial level within 5 min [Figures 1 and 2].
Pheniramine maleate pre-treatment blocked BK-induced changes
Injection of pheniramine maleate (0.11 mg or 0.45 mg/kg) per se did not produce any change in the resting RF, RMV, MAP, and HR up to 20 min. However, pheniramine maleate pre treatment attenuated the tachypneic changes (RF from 90.5 ± 3.63 to 99.8 ± 4.22/min), hyperventilatory changes (RMV from 171.8 ± 7.59 ml/min to 189.5 ± 8.51 ml/min), hypotensive changes (MAP from 90.7 ± 1.94 mmHg to 82.8 ± 2.57 mmHg), and bradycardiac changes (HR from 323.5 ± 6.49 to 315.8 ± 8.3) significantly, produced by BK [Figures 2 and 3]. The changes in these parameters were significantly greater than the time-matched BK only group (P < 0.05, post hoc Dunnett’s t-test [two sided]) or from the initial responses (P < 0.05, Student’s t-test for paired observations).
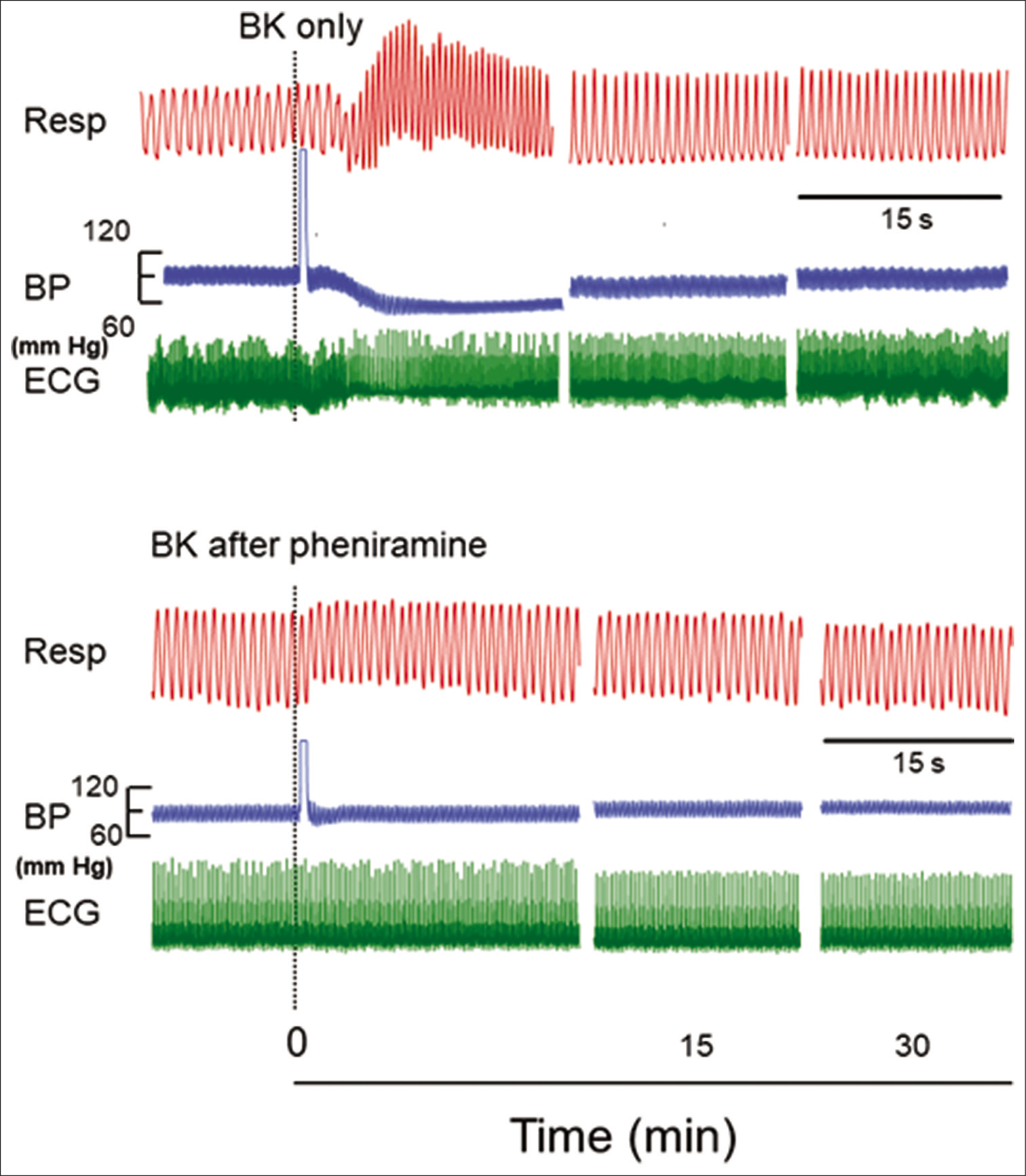
- Original recordings showing the effect of injecting 1 µM concentration of bradykinin (BK) (0.10 ml) in a segment of femoral artery on respiration (Resp), blood pressure (BP), and electrocardiogram (ECG) in the naive and in pheniramine maleate pretreated rats. The responses after BK are shown at different time intervals as indicated in the lower panel. The point of injection is shown by dotted line. The horizontal line between Resp and BP indicates 15 s for all parameters.
DISCUSSION
Intra-arterial administration of BK (BK, a nociceptive agent) produced immediate hyperventilatory, hypotensive, and bradycardiac responses within a latency of 5–8 s. The shorter latencies of responses indicate the neuronal mechanisms in producing the responses. Since the BK is injected in a segment of femoral artery retrogradely; therefore, it is likely that it would remain intravascularly for some time. The femoral artery was also made an end artery by tying the cannula along with the artery which further prevented the spillage of the BK. Moreover, the volumes of all the injectables were kept constant at 0.10 ml to minimize the systemic spillage. Similar results with shorter latency in ventilatory changes are also observed with known nociceptive agents (capsaicin/anandamide) in similar experimental settings elsewhere.[8,13] In another study, it has been shown that intra-arterial injection of BK produced immediate hyperventilatory and hypotensive responses but the HR changes were not observed in their study.[12]
In the present study, the responses were recorded for extended time up to 30 min to visualize the delayed changes in cardiorespiratory parameters. The experimental design was also improvised using the cannulation of single femoral artery only. This cannulation was used for the BP recording as well as for the administration of chemicals (saline/agonist/ antagonist) without stoppage of BP recording as pediatric iv cannula (24 G, double port) with injection valve was used. The interruption in BP recording during drug injection marks the stimulus artifact which interrupts the BP recording hardly for the fraction of a second.
It is well known that intra-arterial injection of a nociceptive agonist causes hyperventilatory and hypotensive responses.[8,10] As B/L vagotomy attenuated the venom-induced bradycardiac responses;[14] therefore, it is possible that the present bradycardiac responses are parasympathetically mediated. However, the precise mechanisms and the involvement of central and peripheral sympathetic reflexes need to be explored.
![Pheniramine maleate (+Phenira) pre-treatment blocked the bradykinin (BK)-induced responses. The time-matched responses relationship in pheniramine maleate pretreated animals and BK only group on respiratory frequency (RF), respiratory minute ventilation (RMV), mean arterial pressure (MAP), and heart rate (HR) is shown. The RF, RMV, MAP, and HR responses are significantly different from BK only group (P < 0.05, post hoc Dunnett’s t-test [two sided]). Dotted line indicates the point of injection of saline (s)/BK. An asterisk (*) indicates P < 0.05 as compared to “BK only” group from pheniramine maleate pre-treated group.](/content/114/2020/64/3/img/IJPP-64-174-g003.png)
- Pheniramine maleate (+Phenira) pre-treatment blocked the bradykinin (BK)-induced responses. The time-matched responses relationship in pheniramine maleate pretreated animals and BK only group on respiratory frequency (RF), respiratory minute ventilation (RMV), mean arterial pressure (MAP), and heart rate (HR) is shown. The RF, RMV, MAP, and HR responses are significantly different from BK only group (P < 0.05, post hoc Dunnett’s t-test [two sided]). Dotted line indicates the point of injection of saline (s)/BK. An asterisk (*) indicates P < 0.05 as compared to “BK only” group from pheniramine maleate pre-treated group.
Involvement of kinin and prostaglandins to noxious stimulation has already been shown by some workers.[15] BK is an inflammatory mediator and produces their actions involving BK 1 (B1) and BK-2 (B2) receptors.[16] The B1 receptors are normally dormant and are expressed after exposure to toxic chemicals/tissue injury products such as cytokines, substance P, neuropeptides, capsaicin, or after repeated exposure to BK.[16] The inflammatory mediators (serotonin, BK, prostaglandins, histamines, etc.) mediate the nociceptive vasosensory reflex responses via the VR1 which is activated by capsaicin and other vanilloid compounds.[17] The VR1 is a ligand gated, non-selective cation channel, expressed predominantly on sensory neurons. VR1 responds to noxious stimuli including capsaicin, heat, and extracellular acidification and it is able to integrate simultaneous exposure to these stimuli.[17-20] BK is known to stimulate the release of histamine from mast cells.[21] Mast cells are also found to be located in the perivascular areas.[22] Thus, it is possible that locally injected BK might have augmented the release of histamine from the perivascular mast cells to mediate the responses through histamine receptors. Our results are consistent with the above findings as pheniramine maleate (H1 and H2 receptor blocker) pre-treatment completely blocked the BK-induced hypotensive responses. In addition, it also blocked the hyperventilatory and bradycardiac responses indicating the role of histamine receptors in modulating the vasosensory reflex responses. These findings also indicate that there is some linkage between the histamine receptors and the BK-receptors in producing the reflex responses. It would have been better to include histamine treated group, that is, histamine + BK antagonist group to demonstrate the cross reactivity, but we could not perform those experiments because of the limitations of our resources.
CONCLUSION
The present results demonstrate that the intra-arterial injection of 1 μM concentration of BK produced immediate hyperventilatory, hypotensive, and bradycardiac responses which were abolished completely in the pheniramine maleate pre-treated group. Our data provide the evidences for the role of H1 and H2 receptors in producing the vasosensory reflex responses. Therefore, it is concluded that BK-induced reflex responses can be modulated by histamine receptor antagonist. However, the precise interaction between bradykinin and histamine receptors requires further investigation.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Peripheral chemoreceptors and cardiovascular regulations. Physiol Rev. 1994;74:543-94.
- [CrossRef] [PubMed] [Google Scholar]
- Functions of afferents in cardiovascular sympathetic nerves. J Auton Nerv Syst. 1981;3:231-6.
- [CrossRef] [Google Scholar]
- Analysis of the effects of intravenously injected capsaicin in the rat. Naunyn Schmiedebergs Arch Pharmacol. 1982;320:54-7.
- [CrossRef] [PubMed] [Google Scholar]
- Capsaicin-induced reflex fall in rat blood pressure is mediated by afferent substance P-containing neurones via a reflex centre in the brain stem. Naunyn Schmiedebergs Arch Pharmacol. 1983;324:293-5.
- [CrossRef] [PubMed] [Google Scholar]
- Activation of P2X receptors for adenosine triphosphate evokes cardiorespiratory reflexes in anaesthetized rats. J Physiol. 1998;359:1-18.
- [CrossRef] [PubMed] [Google Scholar]
- Anandamide induces cardiovascular and respiratory reflexes via vasosensory nerves in the anaesthetized rat. Br J Pharmacol. 2001;134:655-63.
- [CrossRef] [PubMed] [Google Scholar]
- Vasosensory responses elicited by Indian red scorpion venom last longer than the capsaicin-induced responses. Indian J Exp Biol. 2008;46:755-9.
- [Google Scholar]
- Intra-arterial injection of Mesobuthus tamulus venom elicits cardiorespiratory reflexes involving perivascular afferents. Toxicon. 2005;46:820-6.
- [CrossRef] [PubMed] [Google Scholar]
- Injection of Mesobuthus tamulus venom in distal segment of femoral artery evokes hyperventilatory and hypertensive responses in anesthetised rats. Neurosci Lett. 2008a;438:64-6.
- [CrossRef] [PubMed] [Google Scholar]
- Perivascular nerves induce cardiorespiratory reflexes in response to algogens in anaesthetised rats. Neurosci Res. 2004;50:271-81.
- [CrossRef] [PubMed] [Google Scholar]
- Alpha, beta-Methylene ATP elicits a reflex pressor response arising from muscle in decerebrate cats. J Appl Physiol. 2002;93(1985):834-41.
- [CrossRef] [PubMed] [Google Scholar]
- Buthus tamulus venom-induced vasosensory reflexes are mediated through efferent pathways in sympathetic and vagal parasympathetics. Neurosci Lett. 2009;464:199-202.
- [CrossRef] [PubMed] [Google Scholar]
- Role of kinin and prostaglandin in cutaneous thermal nociception. Int Immunopharmacol. 2002;2:2005-12.
- [CrossRef] [Google Scholar]
- The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816-24.
- [CrossRef] [PubMed] [Google Scholar]
- The involvement of bradykinin B1 and B2 receptor mechanisms in cytokine-induced mechanical hyperalgesia in the rat. Br J Pharmacol. 1994;113:63-8.
- [CrossRef] [PubMed] [Google Scholar]
- The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531-43.
- [CrossRef] [Google Scholar]
- Histamine secretion from mast cells stimulated with bradykinin. Agents Actions. 1990;30:67-9.
- [CrossRef] [PubMed] [Google Scholar]
- Platelets trigger perivascular mast cell degranulation to cause inflammatory responses and tissue injury. Sci Adv. 2020;6:eaay6314.
- [CrossRef] [PubMed] [Google Scholar]






